Hauptinhalt
Access to Functionalized Pyrenes, Peropyrenes, Terropyrenes, and Quarterropyrenes via Reductive Aromatization
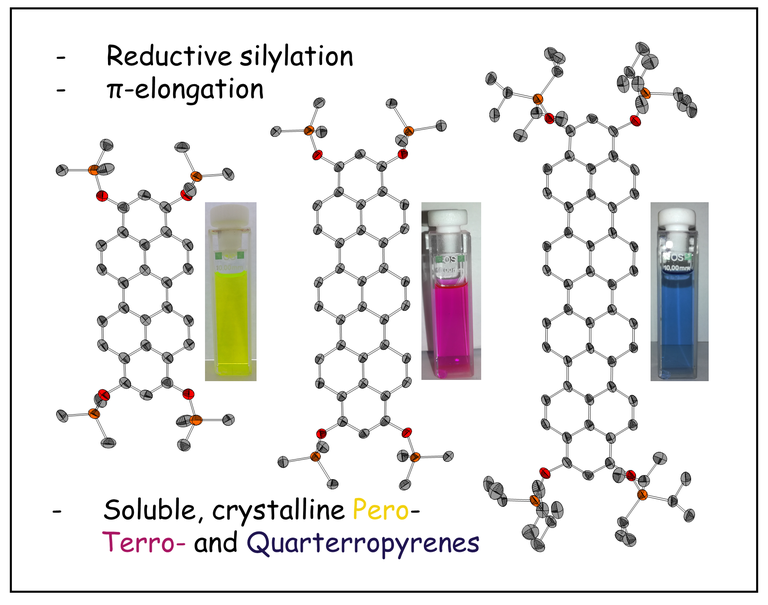
Pyrene has extensively been studied as archetypical fluorophor by synthesis and photophysical research groups. Much less was known for its higher homologues, so called ropyrenes. We developed a modular synthesis strategy building up their molecular backbones and furnishing the target dyes including an unprecedented quarteropyrene via a reductive aromatization step. This series of ropyrene silylethers was systematically studied by XRD, UV-Vis- and fluorescence spectroscopy as well as cyclovoltammetry and (TD-)DFT calculations correlating physical properties and frontier orbitals with their size.
Veröffentlichung: Access to functionalized Pyrenes, Peropyrenes, Terropyrenes and Quarterropyrenes via Reductive Aromatization.
S. Werner, T. Vollgraff, J. Sundermeyer, Angew. Chem. Int. Ed. 2021, 60, 13631–13635; Angew. Chem. 2021, 133, 13743–13748. DOI: 10.1002/anie.202100686; DOI: 10.1002/ange.202100686