Main Content
2015
Inhalt ausklappen Inhalt einklappen J. PHYS. CHEM. C: "Structural Properties of Picene-Perfluoropentacene and Picene-Pentacene Blends: Superlattice Formation versus Limited Intermixing"
J. Dieterle, K. Broch, A. Hinderhofer, H. Frank, J. Novak, A. Gerlach, T. Breuer, R. Banerjee, G. Witte, F. Schreiber
Journal of Physical Chemistry C 119 (47), 26339–26347 (2015), DOI: 10.1021/acs.jpcc.5b08866
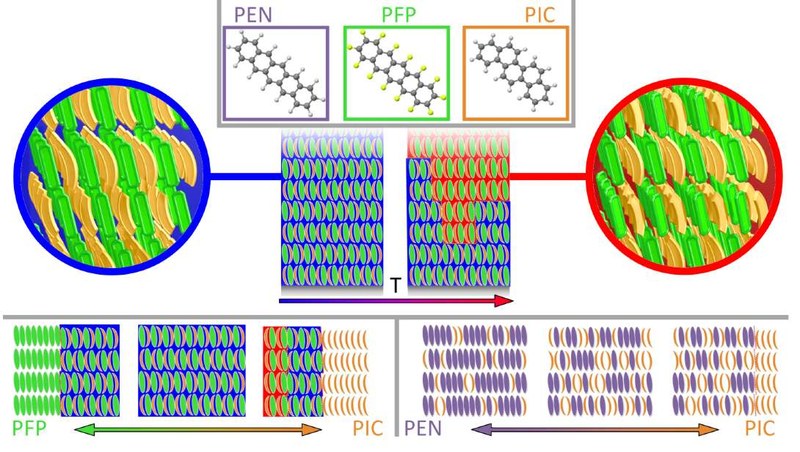
The structure and morphology of mixed thin films of picene (C22H14, PIC) and perfl uoropentacene (C22F14, PFP) as well as mixed thin films of PIC and pentacene (C22H14, PEN) grown by simultaneous co-evaporation is investigated using X-ray di ffraction, atomic force microscopy and near-edge X-ray absorption spectroscopy. For both systems we fi nd mixing on the molecular level and the formation of mixed structures. However, due to the strongly diff erent interactions in both mixtures the ordering is fundamentally ddifferent. For the equimolar PFP:PIC mixtures, we observe the formation of two diff erent mixed polymorphs with unit cells containing 2 PIC and 2 PFP molecules depending on the growth temperature. One of these polymorphs is a superlattice with in-plane compound segregation. The other polymorph is less symmetric and results only in a very short ranged in-plane ordering. In contrast, the PEN:PIC mixtures form crystals with unit cell parameters continuously changing with the molar concentrations between those of the pure compounds. The position of molecular species within the crystal lattice is statistical. Surprisingly, for higher concentrations of PIC we observe phase separation of surplus PIC molecules which corresponds to a limited intermixing of the two compounds. Finally, the results are discussed in the context of other organic semiconductor binary mixtures showing that besides chemical composition and sterical compatibility the intramolecular arrangement of the atoms important for intermolecular interactions signi ficantly infl uences the structure formation in organic semiconductor blends.Inhalt ausklappen Inhalt einklappen CRYST. GROW. DES.: "Polymorph-Selective Preparation and Structural Characterization of Perylene Single-Crystals"
A. Pick, M. Klues, A. Rinn, K. Harms, S. Chatterjee, G. Witte
Crystal Growth & Design 15 (11), 5495-5504 (2015), DOI: 10.1021/acs.cgd.5b01130
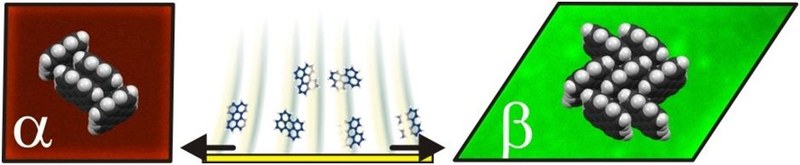
Organic semiconductors occurring in polymorphic structures represent excellent model systems for fundamental studies of optoelectronic excitations in different crystalline configurations. Perylene is an archetypal polycyclic aromatic hydrocarbon appearing in two polymorphs known as α- and β-phases which adopt different molecular packing motifs. However, the growth of high quality single-crystals with appropriate sizes and polymorph selectivity remains challenging. In this study, we compare various approaches towards a polymorph-selective perylene single-crystal growth. Though crystals of both polymorphs are obtained from toluene solution (either by cooling of saturated solution or by evaporation of solvents) they exhibit numerous defects and their size cannot be precisely controlled. Vapor deposition and re-sublimation favors the formation of α-crystals which can be rationalized by a newly identified thin-film phase forms initially. Further, we demonstrate that organic molecular beam deposition onto silicone-oil covered substrates enables the fabrication of high quality crystals of both phases. The relative occurrence of the individual polymorphs is controlled by the actual deposition parameters. Combining the results of X-ray diffraction, atomic-force microscopy, and fluorescence analysis enables an unambiguous polymorph identification solely based on the characteristic crystal shape. The morphological characterization reveals characteristic screw-dislocations at crystals grown from solution or by re-sublimation while the liquid mediated crystals exhibit exceptionally flat surfaces and enable detailed fluorescence studies without defect-related emission signals.Inhalt ausklappen Inhalt einklappen SMALL: "Photo-electrochemical Bioanalysis of Guanosine Monophosphate Using Coupled Enzymatic Reactions at a CdS/ZnS Quantum Dot Electrode"
N. Sabir, N. Khan, J. Völkner, F. Widdascheck, P. del Pino, G. Witte, M. Riedel, F. Lisdat, M. Konrad, W. J. Parak
Small 11 (43), 5844-5850 (2015), DOI: 10.1002/smll.201501883
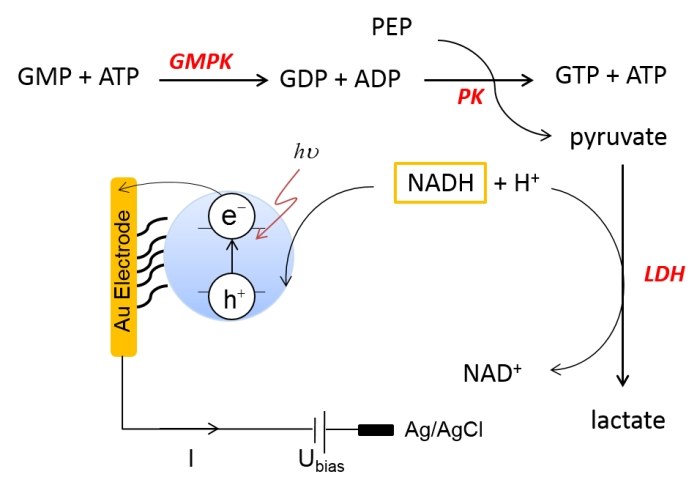
A photo-electrochemical sensor for the specific detection of guanosine monophosphate (GMP) is demonstrated, based on three enzymes combined in a coupled reaction assay. The first reaction involves the adenosine triphosphate (ATP)-dependent conversion of GMP to guanosine diphosphate (GDP) by guanylate kinase, which warrants substrate specificity. The reaction products ADP and GDPare co-substrates for the enzymatic conversion of phosphoenolpyruvate to pyruvate in a second reaction mediated by pyruvate kinase. Pyruvate in turn is the co-substrate for lactate dehydrogenase that generates lactate via oxidation of nicotinamide adenine dinucleotide (reduced form) NADH to NAD+. This third enzymatic reaction is electrochemically detected. For this purpose a CdS/ZnS quantum dot (QD) electrode is illuminated and the photocurrent response under fixed potential conditions is evaluated. The sequential enzyme reactions are first evaluated in solution. Subsequently, a sensor for GMP is constructed using polyelectrolytes for enzyme immobilization.Inhalt ausklappen Inhalt einklappen ACS APPL. MATER. INTERFACES: "Controlling Nanostructures by Templated Templates: Inheriting Molecular Orientation in Binary Heterostructures"
T. Breuer, G. Witte
ACS Applied Materials & Interfaces 7 (36), 20485-20492 (2015), DOI: 10.1021/acsami.5b07409
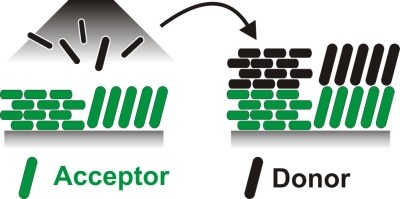
Precise preparation strategies are required to fabricate molecular nanostructures of specific arrangement. In bottom-up approaches, where nanostructures are gradually formed by piecing together individual parts to the final structure, the self-ordering mechanisms of the involved structures are utilized. In order to achieve the desired structures regarding morphology, grain size and orientation of the individual moieties, templates can be applied, which influence the formation process of subsequent structures. However, this strategy is of limited use for complex architectures, as the templates only influence the structure formation at the interface between the template and the first compound. Here, we discuss the implementation of so-called templated templates and analyze, to which extent orientations of initial layers are inherited in top layers of another compound to enable structural control in binary heterostructures. For that purpose we have prepared crystalline templates of the organic semiconductors pentacene and perfluoropentacene in different exclusive orientations. We observe that for templates of both individual materials the molecular orientation is inherited in the top layers of the respective counterpart. This behavior is also observed for various other molecules indicating the robustness of this approach.Inhalt ausklappen Inhalt einklappen JES: "Characterization of Orientational Order in π-conjugated Molecular Thin Films by NEXAFS"
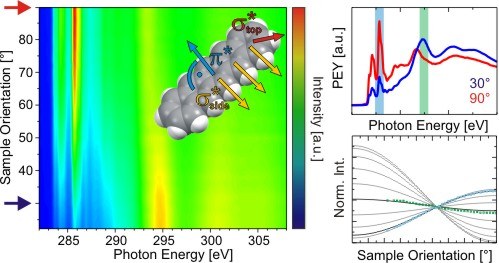
T. Breuer, M. Klues, G. Witte
Journal of Electron Spectroscopy 204 (A), 102-115 (2015), DOI: 10.1016/j.elspec.2015.07.011
Enabled by the improved availability of synchrotron facilities, near-edge x-ray absorption fine structure (NEXAFS) spectroscopy has become a widely used technique, especially due to its tunable, potentially very high, surface-sensitivity and its capability of analyzing the electronic structure of unoccupied orbitals. In this article we describe the fundamentals and technical requirements for NEXAFS spectroscopy with special focus on its application to the structural characterization of organic thin films. Based on prominent examples we discuss typical experimental applications of this technique and their characteristics compared to complementary methods. Since the evaluation of NEXAFS measurements is not straight-forward and allows for objectionable misinterpretations, we discuss numerous parasitic and often unattended effects which complicate the reliable analysis of NEXAFS spectra. Especially for the case of orientation determinations by means of NEXAFS using dichroisms analyses, the effects of molecular geometry and crystal packing motifs are elucidated in detail to provide a comprehensive picture on potential obstacles which often occur during the study of organic thin films.Inhalt ausklappen Inhalt einklappen CHEM.: "Efficient Syntheses of Novel Fluoro-Substituted Pentacenes and Azapentacenes: Molecular and Solid-State Properties"
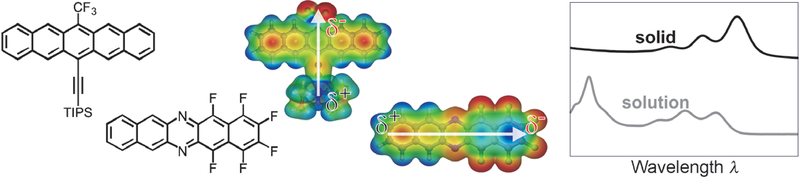
J. Schwaben, N. Münster, M. Klues, T. Breuer, P. Hofmann, K. Harms, G. Witte, U. Koert
Chemistry - A European Journal 21 (39), 13758-13771 (2015), DOI: 10.1002/chem.201501399
Non-symmetrical 6,13-disubstituted pentacenes bearing trifluoromethyl and aryl substituents have been synthesized starting from pentacenequinone. Diazapentacenes with a variety of fluorine substituents were prepared either via a Hartwig–Buchwald aryl amination route or by a SNAr strategy. As a result of a non-symmetric substitution pattern containing electron-donating substituents in combination with electron-accepting fluorine substituents, the synthesized compounds feature distinct molecular dipoles. All compounds are analyzed regarding their optoelectronic properties in solution with special focus on the frontier orbital energies as well as their molecular packing in the crystal structures. The analyses of isolated molecules are complemented by thin-film studies to examine their solid-state properties. A precise comparison between these and the molecular properties gave detailed insights into the exciton binding energies of these compounds, which are explained by means of a simple model considering the molecular packing and polarizabilities.Inhalt ausklappen Inhalt einklappen CRYST. GROW. DES.: "Epitaxial TTF-TCNQ Thin Films on KCl(100): New Preparation Methods and Observation of Interface-Mediated Thin Film Polymorph"
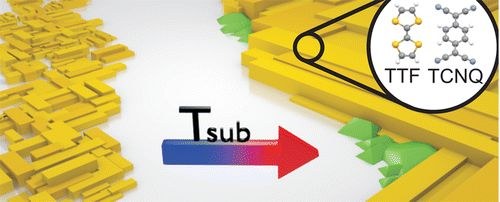
A. Mänz, T. Breuer, G. Witte
Crystal Growth & Design 15 (1), 395–403 (2015), DOI: 10.1021/cg501484p
Combining organic compounds of complementary ionization potential and electron affinity allows to fabricate charge-transfer complexes which exhibit remarkable properties, resulting e.g. in very high conductivity. Though the bulk properties of the prototypical organic conductor Tetrathiafulvalene-Tetracyanoquinodimethane (TTF-TCNQ) have been studied in detail, the influence of defects and crystallite size on resulting electronic properties as well as an integration of these materials in organic thin film devices is barely explored. One important requirement for such a comprehension is the precise control over crystallite size and quality. In this study, we report on different strategies to prepare crystalline TTF-TCNQ thin films and compare their structural quality. While conventional organic molecular beam deposition of TTF-TCNQ onto KCl(100) substrates enables the growth of epitaxial thin films with grain dimensions of up to 2 μm, further enhancement of the crystallite dimensions by raising the growth temperature is thermally limited by vanishing sticking and onset of vaporization. Using more sophisticated methods like hot wall evaporation, however, allows to overcome these limitations and yields crystalline islands with extensions enhanced by two orders of magnitude. Furthermore, we identify and provide a full structure solution of a yet unknown interface-mediated thin film polymorph of TTF-TCNQ, which is adopted in films of thicknesses below 1 μm.