Main Content
2022
Inhalt ausklappen Inhalt einklappen NAT. SCI., "Singlet-exciton optics and phonon-mediated dynamics in oligoacene semiconductor crystals"
J. J. P. Thompson, D. Muth, S. Anhäuser, D. Bischof, M. Gerhard, G. Witte, E. Malic
Nat. Sci, e20220040 (2022), • DOI: 10.1002/ntls.20220040
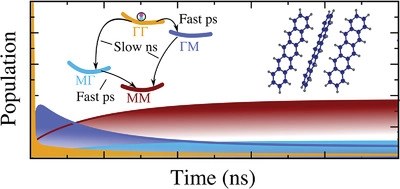
Organic semiconductor crystals stand out as an efficient, cheap, and diverse platform for realizing optoelectronic applications. The optical response of these crystals is governed by a rich tapestry of exciton physics. So far, little is known on the phonon-driven singlet-exciton dynamics in this class of materials. In this joint theory–experiment work, we combine the fabrication of a high-quality oligoacene semiconductor crystal and characterization via photoluminescence measurements with a sophisticated approach to the microscopic modeling in these crystals. This allows us to investigate singlet-exciton optics and dynamics. We predict phonon-bottleneck effects in pentacene crystals, and find that dark excitons act as crucial phonon-mediated relaxation scattering channels in both pentacene and tetracene. While the efficient singlet fission in pentacene crystals hampers the experimental observation of this bottleneck effect, we reveal both in theory and experiment a distinct polarization and temperature dependence in absorption and photoluminescence spectra of tetracene crystals, including microscopic origin of exciton linewidths, the activation of the higher Davydov states at large temperatures, and polarization-dependent quenching of specific exciton resonances. Our joint theory–experiment study represents a significant advance in microscopic understanding of singlet-exciton optics and dynamics in oligoacene crystals.Inhalt ausklappen Inhalt einklappen CRYST. GROWTh DES., "Solvent Polarity Influenced Polymorph Selection of Polar Aromatic Molecules"
Daniel Bischof, Matthias W. Tripp, Sergei I. Ivlev, Ulrich Koert, and Gregor Witte
Cryst Growth Des., 22, 12, 6857–6862 (2022), • Doi: 10.1021/acs.cgd.2c01069
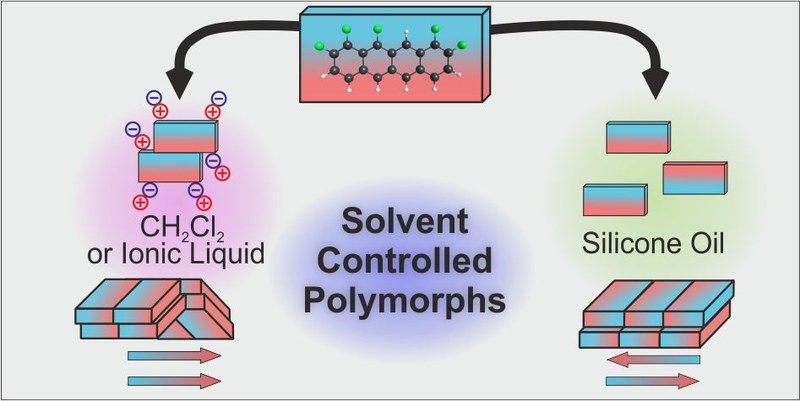
The crystallization and polymorph control of rigid polar molecules by solvent polarity was investigated for the case of 1,2,9,10,11-pentafluorotetracene (F5TET), an extended π-conjugated molecule with large in-plane dipole moment. Liquid assisted crystallization from polar solvents results in a dipole-parallel molecular arrangement with a crisscross packing. By contrast, nonpolar solvents lead to a more stable polymorph, which exhibits a dipole-antiparallel arrangement with a slip-stacked packing. Since for both polymorphs no solvents are incorporated in the crystal lattice, we attribute the different growth modes to a screening of the electrostatic forces by the solvent during nucleation. The results emphasize that solvent polarity must be considered when exploring the polymorphic landscape of molecular materials, which is particularly important for organic semiconductors that typically consist of π-conjugated molecules with rather low solubility, thus hampering normal solution crystallization.Inhalt ausklappen Inhalt einklappen ACS Appl. Mater Interfaces, "F-Center-Mediated Growth of Patterned Organic Semiconductor Films on Alkali Halides"
Darius Günder, Valentin Diez-Cabanes, Andrea Huttner, Tobias Breuer, Vincent Lemaur, Jérôme Cornil, and Gregor Witte*Gregor Witte
ACS Appl. Mater. Interfaces, 14, 40, 46086-46094 (2022), • Doi: 10.1021/acsami.2c13934
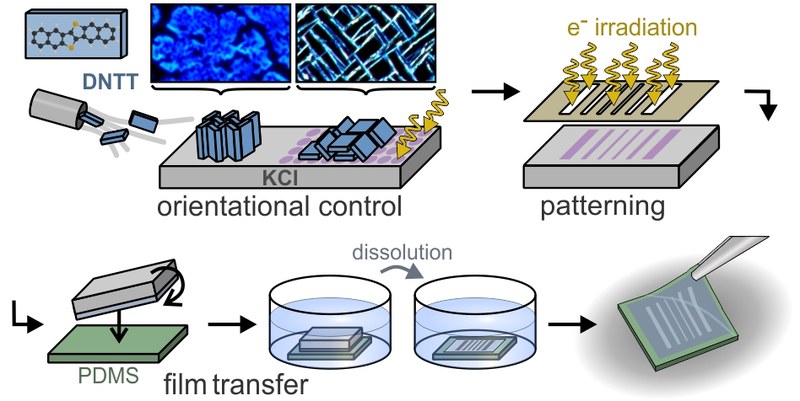
Organic semiconductors combine flexible tailoring of their optoelectronic properties by synthetic means with strong light–matter coupling, which is advantageous for organic electronic device applications. Although spatially selective deposition has been demonstrated, lateral patterning of organic films with simultaneous control of molecular and crystalline orientation is lacking as traditional lithography is not applicable. Here, a new patterning approach based on surface-localized F-centers (halide vacancies) generated by electron irradiation of alkali halides is presented, which allows structural control of molecular adlayers. Combining optical and atomic force microscopy, X-ray diffraction, and density functional theory (DFT) calculations, it is shown that dinaphthothienothiophene (DNTT) molecules adopt an upright orientation on pristine KCl surfaces, while the F-centers stabilize a recumbent orientation, and that these orientations are maintained in thicker films. This specific nucleation results also in different crystallographic morphologies, namely, densely packed islands and jagged fibers, each epitaxially aligned on the KCl surface. Spatially selective surface irradiation can also be used to create patterns of F-centers and thus laterally patterned DNTT films, which can be further transferred to any (including elastomer) substrate due to the water solubility of the alkali halide growth templates.Inhalt ausklappen Inhalt einklappen LANGMUIR: "Template and Temperature-Controlled Polymorph Formation in Squaraine Thin Films"
Frank Balzer, Tobias Breuer, Gregor Witte, and Manuela Schiek*
Langmuir, 38, 9266−9277 (2022), • Doi:
10.1021/acs.langmuir.2c01023
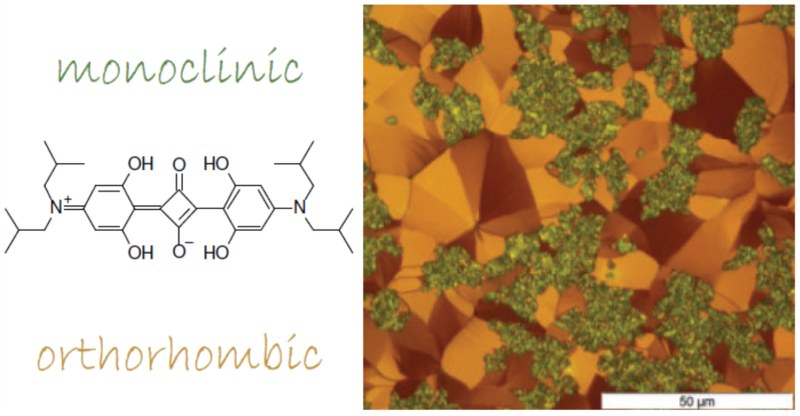
Controlling the polymorph formation in organic semiconductor thin films by the choice of processing parameters is a key factor for targeted device performance. Small molecular semiconductors such as the prototypical anilino squaraine compound with branched butyl chains as terminal functionalization (SQIB) allow both solution and vapor phase deposition methods. SQIB has been considered for various photovoltaic applications mainly as amorphous isotropic thin films due to its broad absorption within the visible to deep-red spectral range. The two known crystalline polymorphs adopting a monoclinic and orthorhombic crystal phase show characteristic Frenkel excitonic spectral signatures of overall H-type and J-type aggregates, respectively, with additional pronounced Davydov splitting. This gives a recognizable polarized optical response of crystalline thin films suitable for identification of the polymorphs. Both phases emerge with a strongly preferred out-of-plane and rather random in-plane orientation in spin-casted thin films depending on subsequent thermal annealing. By contrast, upon vapor deposition on dielectric and conductive substrates, such as silicon dioxide, potassium chloride, graphene, and gold, the polymorph expression depends basically on the choice of growth substrate. The same pronounced out-of-plane orientation is adopted in all crystalline cases, but with a surface templated in-plane alignment in case of crystalline substrates. Strikingly, the amorphous isotropic thin films obtained by vapor deposition cannot be crystallized by thermal postannealing, which is a key feature for the spin-casted thin films, here monitored by polarized in situ microscopy. Combining X-ray diffraction, atomic force microscopy, ellipsometry, and polarized spectro-microscopy, we identify the processing-dependent evolution of the crystal phases, correlating morphology and molecular orientations within the textured SQIB filmsInhalt ausklappen Inhalt einklappen Adv. Mater. Interfaces, "On the Role of Collective Electrostatic Effects in Electronic Level Pinning and Work Function Changes by Molecular Adlayers: The Case of Partially Fluorinated DNTTs Adsorbed Flat-Lying on Various Metals and Hetero-Structures"

Maximilian Dreher, David Cornil, Matthias W. Tripp, Ulrich Koert, Jérôme Cornil and
Gregor Witte.
Adv. Mater. Interfaces 2200361 (2022), • Doi:
10.1002/admi.202200361
Modifying the work function of metal electrodes by monolayers of molecules with
specifically tailored electronic properties is a versatile tool, but such chemical
modifications often also affect the adsorption geometry and packing density,
making microscopic modeling difficult. Using scanning tunneling microscopy, it
is shown that the recently synthesized partially fluorinated dinaphthothienothiophenes
(DNTTs) adopt the same interface structure on different metal substrates
independent of the degree of fluorination. Combining Kelvin probe measurements
and density functional theory (DFT) calculations, a highest occupied molecular
orbital (HOMO) pinning effect for such FxDNTTs on Au(111) and Ag(111)
induced by collective electrostatic interactions in the monolayer is observed.
Since the adsorption of weakly interacting molecules such as the FxDNTTs is not
restricted to specific surfaces as is the case with SAMs, this concept is extended
to metal substrates with quite different work function values. For a low work
function surface such as Cs(110), a lowest unoccupied molecular orbital (LUMO)
pinning effect is predicted at the theoretical level. Since such alkali metal surfaces
are not experimentally accessible, a well-defined Cs monolayer on Cu(100) as a
low work function substrate is used instead. For this substrate, however, a variation
is observed in the LUMO energies and the work function as a function of the
degree of fluorination. This is attributed to the formation of a second interface
dipole at the buried Cs/Cu interface, which is modulated with the degree of
fluorination and competes with the dipole at the outer molecule/Cs interface.
Such a second internal interface dipole, which can be modified by the top layer,
has to be considered when going to more complex heterointerfaces.Inhalt ausklappen Inhalt einklappen CHEM. EUR. J., "Regioselective Fluorination of Acenes: Tailoring of Molecular Electronic Levels and Solid State Properties"
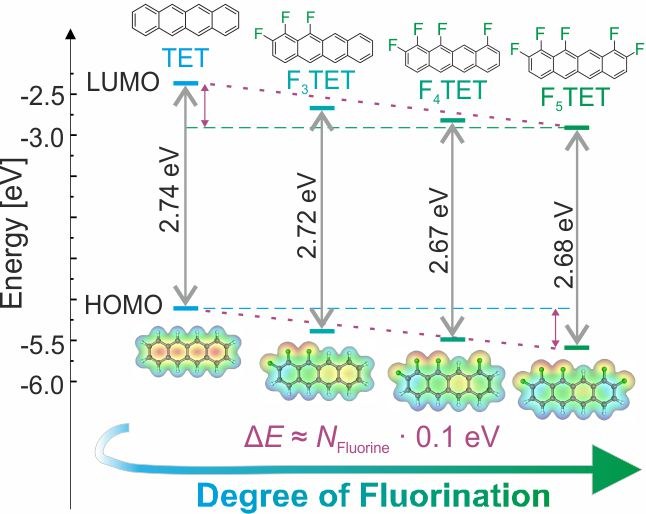
Daniel Bischof, Matthias W. Tripp, Philipp E. Hofmann, Chun-Ho Ip, Sergei I. Ivlev, Marina Gerhard, Ulrich Koert and Gregor Witte
CHEM. EUR. J., e202103653 (2022), • Doi: 10.1002/chem.202103653
Optoelectronic properties of molecular solids are important for organic electronic devices and are largely determined by the adopted molecular packing motifs. In this study we analyzed such structure-property relationships for the partially regioselective fluorinated tetracenes 1,2,12-trifluorotetracene, 1,2,10,12-tetrafluorotetracene and 1,2,9,10,11-pentafluorotetracene that were further compared with tetracene and perfluoro-tetracene. Quantum chemical DFT calculations in combination with optical absorption spectroscopy data show that the frontier orbital energies are lowered with the degree of fluorination, while their optical gap is barely affected. However, the crystal structure changes from a herringbone packing motif of tetracene towards a planar stacking motif of the fluorinated tetracene derivatives, which is accompanied by the formation of excimers and leads to strongly red-shifted photolumin with larger lifetimes.