Main Content
Chemistry of 3-Fluoroallyl Compounds
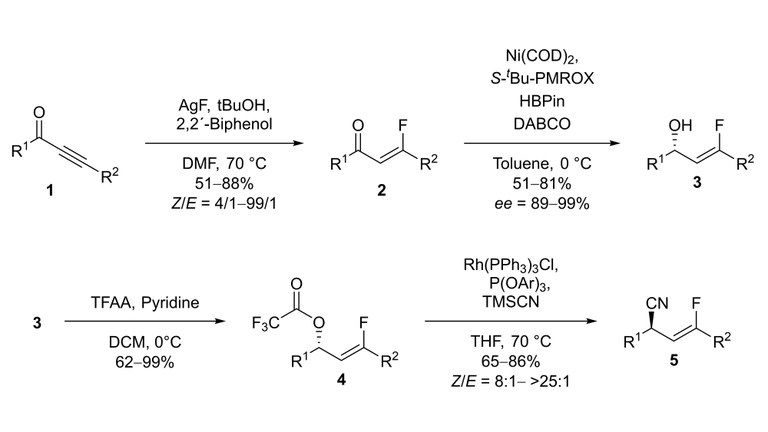
The synthesis of chiral organofluorine compounds is of great interest for pharmaceuticals, agrochemicals and material science due to the key physicochemical properties of fluorine. A hydrofluorination of enolizable ynones 1 with good Z/E selectivity and high functional group tolerance was developed. The asymmetric reduction of the resulted β-fluoroenones 2 was performed in high yield and excellent stereoselectivity.[1] The obtained chiral 3-fluoroallylic alcohols 3 were converted into the 3-fluoroallylic nitriles 5 over two steps by a rhodium-catalyzed allylic cyanation, which exhibits an exclusive regioselectivity, a good functional group tolerance and high Z/E-selectivity.[2]
[1] L. Zygalski, C. Middel, K. Harms, U. Koert “Enolizable β-Fluoroenones: Synthesis and Asymmetric 1,2-Reduction“, Org. Lett. 2018, 20, 5071–5074.
[2] C. Middel, L. Zygalski, J. Meinecke, U. Koert “Rhodium-Catalyzed Regioselective 3-Fluoroallylic Cyanation”, Adv. Synth. Catal. 2021, ASAP.