Main Content
vic-Tricarbonyl Compounds as Intermediates in Organic Synthesis
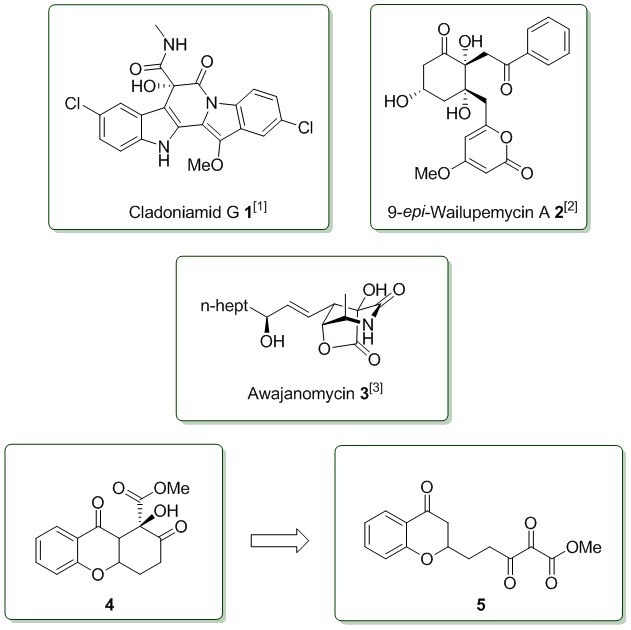
vic-Polycarbonyl derivatives are valuable intermediates in organic synthesis due to their high electrophilicity. Therefore structural motives with a high density of functional groups are accessible in a low number of steps. So far, natural products like Cladoniamide G (1)[1], 9-epi-Wailupemycin A (2)[2] and Awajanomycin (3)[3] could be synthesized using vic-tricarbonyl compounds.[4] A current research project in the Koert-Group aims towards the use of these reactive intermediates in the synthesis of chromone-based, bioactive natural products. The key aspect of this approach is the formation of highly oxidized 5- and 6-membered rings (4→5).
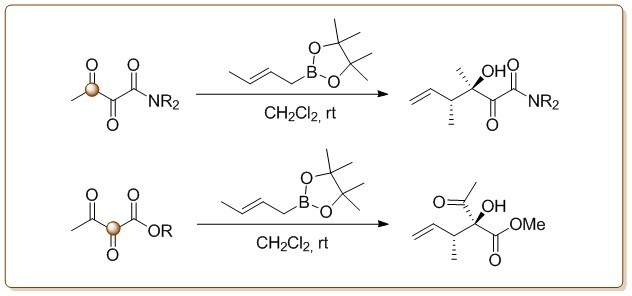
Beside natural product synthesis, development of new organic reactions of vic-diketoamides and –esters are also important subjects. Reactions such as the regio- and diastereoselective crotoborylation[5] (6→7), the passerini-knoevenagel reaction[6] or the addition of mixed alkenyl-dialkyl zincates[7] to vic-tricarbonyl compounds have been subjects of synthetic effort in recent years.
[1] M. Wohlfahrt, K. Harms, U. Koert, Angew. Chem. Int. Ed. 2011, 50, 8404-8406.
[2] J. Schütte, F. Kilgenstein, M. Fischer, U. Koert, Eur. J. Org. Chem. 2014, 5302-5311.
[3] T. Seitz, K. Harms, U. Koert, Synthesis 2014, 46, 381-386.
[4] L. Selter, L. Zygalski, E. Kerste, U. Koert, Synthesis 2017, 49, 17-28.
[5] J. Roßbach, J. Baumeister, K. Harms, U. Koert, Eur. J. Org. Chem. 2013, 662-665.
[6] J. Roßbach, K. Harms, U. Koert, Eur. J. Org. Chem. 2014, 993-1006.
[7] L. Selter, K. Harms, U. Koert, Eur. J. Org. Chem. 2017, 1215-1230.