Main Content
Diffusion, Sorption, and Reaction in Mesopore Networks
The nature of diffusion and accompanying mass transfer resistance are intrinsic to the dynamics at and near solid-liquid interfaces. It addresses a variety of materials and processes in nature and technology, including heterogeneous catalysis, the performance of electrodes, chemical sensing, biomolecular interactions with membranes and cells, as well as adsorption and separation in liquid chromatography.
The efficiency of a process is often controlled by the spatiotemporal distribution and diffusion of molecular species in microporous, mesoporous, or hierarchically structured pore environments. Hierarchically structured materials typically consist of spatial domains characterized by different pore space morphology. For example, with macroporous–mesoporous materials used in separation and catalysis this results in specific transport properties of the different domains, e.g., in the domination of diffusion in the mesopores, in contrast to coupled advective–diffusive transport in the macropores. Molecules reach an interaction site on the surface of the mesopores (e.g., for adsorption or reaction) through diffusion only, which may ultimately run into a diffusion-limited process dynamics and thus limit the overall efficiency.
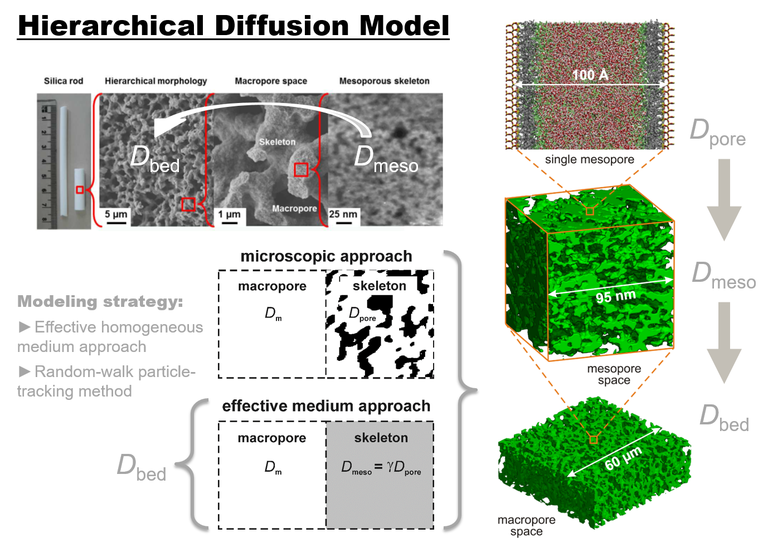
The precise determination of solvent structure and solute mobility at and near the surface of the mesopores as well as the complementary knowledge of the 3D mesopore space morphology forms a prerequisite for the accurate characterization and optimization of diffusive transport properties. To achieve that goal, we established a multiscale simulation approach, which accounts for surface chemistry and solvent structure/solute mobility on the single-pore level as well as pore space morphology on a mesoscopic scale.
First, diffusion at and near model mesopore surfaces is spatiotemporally resolved using molecular dynamics (MD) simulations. Pore-level diffusion is then traced up to the macroscopic level of a material using a hierarchical diffusion model. Using solute molecules that can be distinguished regarding their size, shape, and polarity, these MD simulations provide key data about local concentrations, residence times, diffusive mobilities, as well as data on differential partitioning and adsorption on the pore level. They can rationalize the macroscopic, experimentally accessible transport dynamics and adress effects like retention and selectivity as well as the shape of adsorption isotherms, or even allow to predict these characteristics resulting from a (non)linear thermodynamics. The MD simulation-based effective pore diffusion coefficient Dpore is subsequently combined with physical reconstructions of mesopore and macropore spaces from hierarchically structured materials. It allows us to analyze – through mass transport simulations based on a random-walk approach (Brownian dynamics) – effective diffusion in the interconnected mesopore space (Dmeso) and finally reach effective diffusion at the macroscopic level of a macroporous–mesoporous material (Dbed), e.g., a packing of mesoporous particles or a monolith.
The hierarchical diffusion approach guarantees that relevant molecular details regarding surface modification and solvent/solute properties as well as the resulting phenomena (diffusion into and near the surface modification; adsorption and partitioning under nonlinear conditions) are properly accounted for on the pore level and can be morphologically traced up to the macroscopic field scale, where the data can challenge experiments (and vice versa). Investigated aspects include
(i) the size, shape, polarity, and density of the chemical surface modification,
(ii) the impact of using binary and ternary solvents as well as ions on solvent composition, structure, and mobility near the surface,
(iii) the influence of molecular properties on solute adsorption/partitioning behaviour,
(iv) mean size, size distribution, and heterogeneity of the mesopores, and
(v) the ratio of solute size to mean mesopore size regarding its impact on the actually accessible pore network and resulting effective diffusivities.
► Chemistry is accounted for (surface modification, analyte structure, solvent mixtures)
► Realistic molecular orientation, solvation, distribution, and mobility
► Adsorption, partitioning, and transport emerge from molecular-level picture
Effective diffusion on mesopore and macropore scales of a hierarchically structured monolith or packed bed are subsequently obtained by direct (pore-scale) simulations combined with an effective homogeneous medium approach.
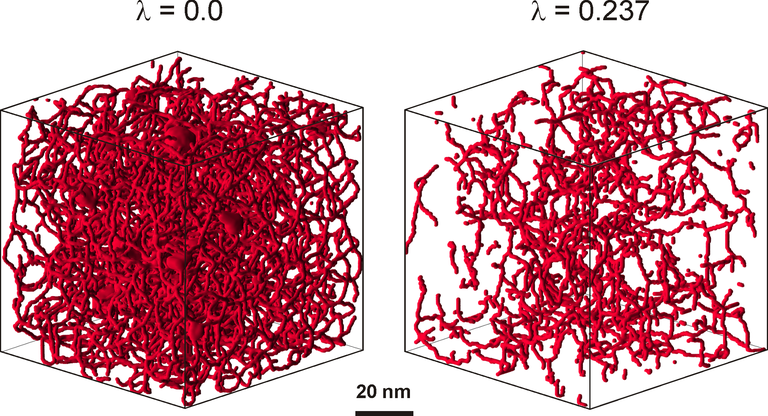
Figure 2: Visualization of the accessible pore network in physically reconstructed mesopore space from the skeleton of a macroporous-mesoporous silica monolith for point-like tracers (left panel) and for finite-size tracers characterized by a ratio of tracer size to mean pore size of 0.237 (right panel).
Highlighted Publications:
- D. Hlushkou, A. Svidrytski, U. Tallarek
Tracer-size-dependent pore space accessibility and long-time diffusion coefficient in amorphous, mesoporous silica.
Journal of Physical Chemistry C 2017, 121, 8416–8426. DOI: 10.1021/acs.jpcc.7b00264
- J. Rybka, J. Kärger, U. Tallarek
Single-molecule and ensemble diffusivities in individual nanopores with spatially dependent mobility.
ChemPhysChem 2017, 18, 2094–2102. DOI: 10.1002/cphc.201700231
- D. Hlushkou, F. Gritti, G. Guiochon, A. Seidel-Morgenstern, U. Tallarek
Effect of adsorption on solute dispersion: A microscopic stochastic approach.
Analytical Chemistry 2014, 86, 4463–4470. DOI: 10.1021/ac500309p
- S.M. Melnikov, A. Höltzel, A. Seidel-Morgenstern, U. Tallarek
How ternary mobile phases allow tuning of analyte retention in hydrophilic interaction liquid chromatography.
Analytical Chemistry 2013, 85, 8850–8856. DOI: 10.1021/ac402123a
- D. Hlushkou, F. Gritti, A. Daneyko, G. Guiochon, U. Tallarek
How microscopic characteristics of the adsorption kinetics impact macroscale transport in chromatographic beds.
Journal of Physical Chemistry C 2013, 117, 22974–22985. DOI: 10.1021/jp408362u