Main Content
Electrochemically mediated seawater desalination
Electrochemically mediated desalination (EMD) of seawater, a new membraneless, energy efficient desalination method, relies on the oxidation of chloride ions, which generates an ion depletion zone and local electric field gradient near the junction of a microchannel branch to redirect sea salt into the brine stream, consequently producing desalted water. The results obtained with the developed model to simulate seawater desalination in the EMD device are close to experimental findings, which represent similar operating conditions. The strength of the numerical implementation of the developed model is in its ability to perform efficient optimization of the geometrical configuration and operating conditions in the EMD unit to increase its efficiency.
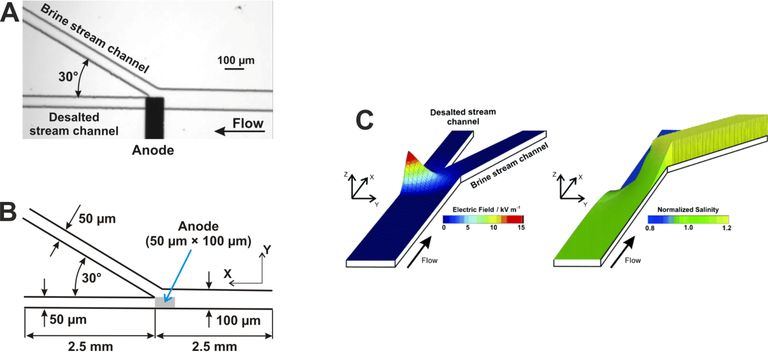
Figure 1: (A) Optical micrograph of the region of a PDMS/glass desalination device near an embedded Pt electrode (anode). (B) Schematic of the microchannel system used for simulation of the EMD device. The thickness (Z-dimension) of the microchannels is 22 µm. (C) Distributions of the axial electric field (left) and normalized salinity (right) in the central plane (z = 11 µm) of the device, simulated for a 0.55 M NaCl solution with a total pressure-driven flow rate of 0.1 μL min−1, a current through the electrode of 50 nA, and an electrode potential of 0.9 V. Corresponding simulated salinity distribution normalized to the inlet value (0.55 M) showing a 20% decrease in salt concentration in the desalted stream.