Main Content
Paper on "Ion Molecule Reactions in the HBr+ + CH4 System: A combined experimental and theoretical study" published in PCCP
D. Plamper, A. Vincent, K. Fujioka, R. Sun, and K.-M. Weitzel
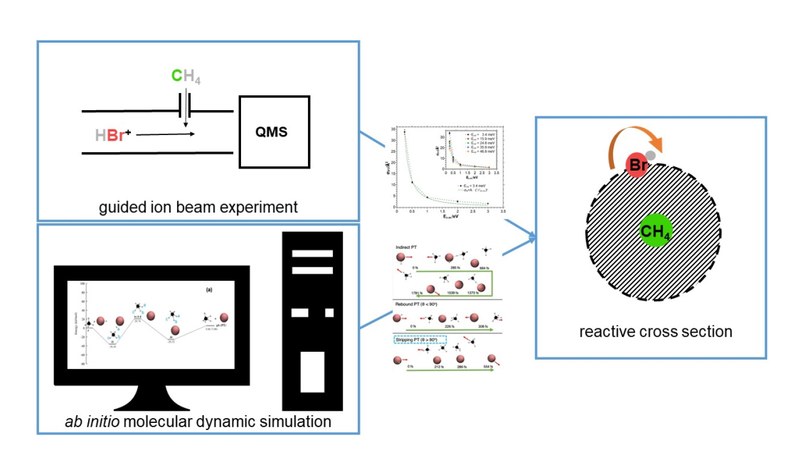
Reactions in the system HBr+ + CH4 have been investigated inside a guided ion-beam apparatus under single-collision conditions. The HBr+ is vibrational and rotational state selected in the electronic X 2Π1/2 state created by (2+1)-REMPI. Due to the exitation scheme employed different rotational states of the HBr+ are accessible. Four reaction channels have been observed. The cross section, σ, for the exothermic proton transfer channel (PT) decreases with increasing collision energy, steeper than predicted by the Langevin model. The cross section also decreases with increasing rotational energy in the HBr+ , with the effect of the rotational energy being stronger than that of translational energy. The cross section for the endothermic charge transfer (CT) increased with increasing collision energy. The energy dependence is well reproduced by a simple line of center (loc) model. Although the bromine transfer (BT) is exothermic the observed cross section increased with increasing collision energy due to an activation barrier on the potential energy surface (PES). Analysis by a modified loc model suggest the relevance of an angle dependence of σ. The cross section for the endothermic hydrogen atom abstraction (HA) exhibits a maximum at 2 eV Ec.m.. The measured cross sections are rationalized by means of reaction dynamics simulations which shows good agreement with the experimental cross sections. The dynamics simulations are carried out with a machine learning potential that is developed and benchmarked with ab initio molecular dynamics simulation. The absolute cross sections predicted by reaction dynamics simulations are well within the same order of magnitude while reproducing the trends over three different collision energies for all four reaction channels. Furthermore, the simulations demonstrate various reaction mechanisms for these reaction channels, including a very interesting HBr+ orientation selectivity for the BT reaction channel.
Phys. Chem. Chem. Phys. 26, 16732, (2024)
Doi: https://doi.org/10.1039/D4CP01121J