Main Content
Energy landscapes, structure and function
In many technically relevant chemical processes a macroscopic variation of the composition, i.e. the local stoichiometry, is operative. This is e.g. the case for the lithiation / delithiation cycling in lithium ion batteries. For a quantitative understanding of the underlying transport processes one would like to know whether the energy required for taking out e.g. one Li+ ion is different for the first as compared to the last ion. This is nothing else but asking for the energy spread of sites occupied in a solid-state material. Unfortunately there no direct measurements for this quantity. Theoretical estimates predict values between 0.1 eV and 1 eV for amorphous samples.
We have recently developed a concept for deriving the complete energy distribution of populated sites (PSED) from the analysis of concentration depletion / replacement zones in our foreign ion charge attachment induced transport (CAIT) experiment. The concept has been described in Materials Today Physics [1].
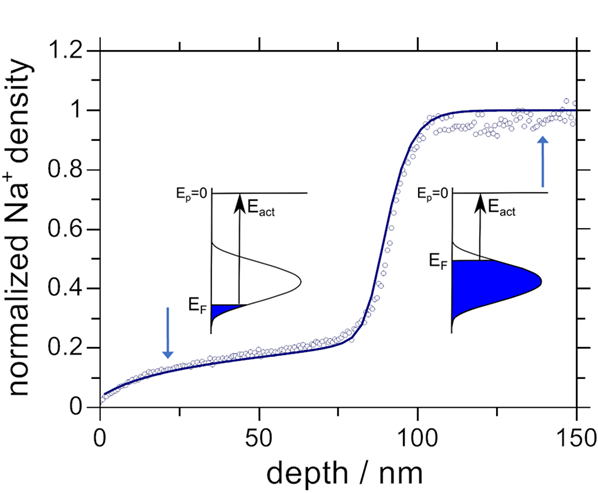
Figure 1 Graphical abstract sketching the sensitivity of a concentration depletion profile to the concentration dependence of the ionic Fermi energy. In the example described the FWHM of the PSED was 0.28 eV.
More recently, we have quantitatively analyzed the site energy distribution of sodium ions in a sodium rubidium borate glass. We demonstrated that external Rb+ ions were exclusively talking to native Na+ ions in the glass leading to the replacement of the latter. The full width at half maximum of the populated part of this SED was found to be 0.32 eV. The mechanism involves Na+ sites being vacated top-down and being filled by Rb+ also top down. Therefore, the Fermi energy of Na+ ions decreases with ongoing experiment, while that of the Rb+ ions stays constant [2].
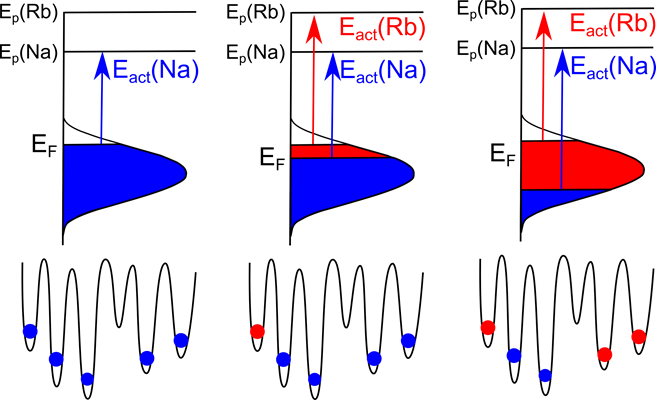
Figure 2 Site energy distribution for Rb+ and Na+ during the CAIT experiment pertinent to the initial Na+ sites, illustrating that Eact(Na) does change but Eact(Rb) remains constant
The CAIT experiment providing access to the populated part of the site energy distribution of ions in a solid state materials is key aspect of the recently installed research unit FOR 5065, “Energy Landscapes and Structure in Ion Conducting Solids”.
Literature:
[1] M. Schäfer, K.-M. Weitzel
Site energy distributions of ions in the potential energy landscape of amorphous solids
Material Today Physics, 5, 12-19, (2018)
[2] Martin Schäfer, David Budina and Karl-Michael Weitzel
Site energy distribution of sodium ions in a sodium rubidium borate glass
Phys.Chem.Chem.Phys. , 21, 26251, (2019)