Main Content
Electron – CAIT studies of ionic and mixed ionic electronic conductivity
The original work on CAIT employed a cation beam of alkali elements. For studying alkali ion transport this is the obvious choice. There are several reasons for being interested in a version of CAIT which is based on electron attachment. For readers with the cation-CAIT approach it is clear that electron attachment will lead to a negative charge-up of the sample surface. Consequently, positive mobile charge carriers inside the sample will move towards the front, but negative mobile charge carriers (be it electrons or anions) will move towards the back.
We have now implemented electron-CAIT employing a standard Erdman-Zipf design as illustrated in Fig. 1.
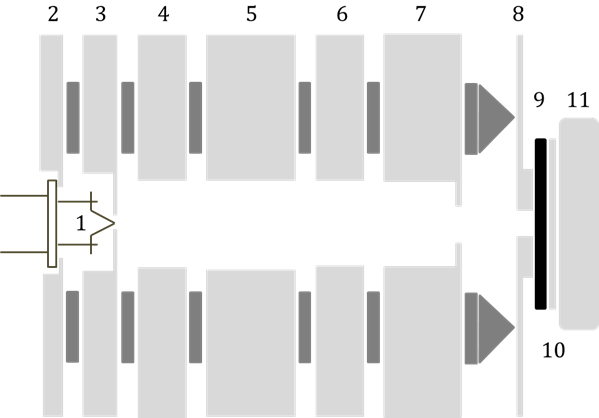
Fig. 1 Scheme of the electron source for use in e-CAIT (Hein et al. submitted for publication).
To test the viability of electron-CAIT (e-CAIT) we have studied the conductivity of a glass standard, i.e. D263T. This is a mixed alkali borosilicate glass, which from several detailed studies in our group is well known to be Na+ and K+ conducting [1]. The absolute ionic conductivity obtained by e-CAIT is shown in Fig. 2. For comparison data from an independent K+-CAIT experiment are also included. Obviously the absolute conductivities as well as the activation energy for ion hopping agree very well.
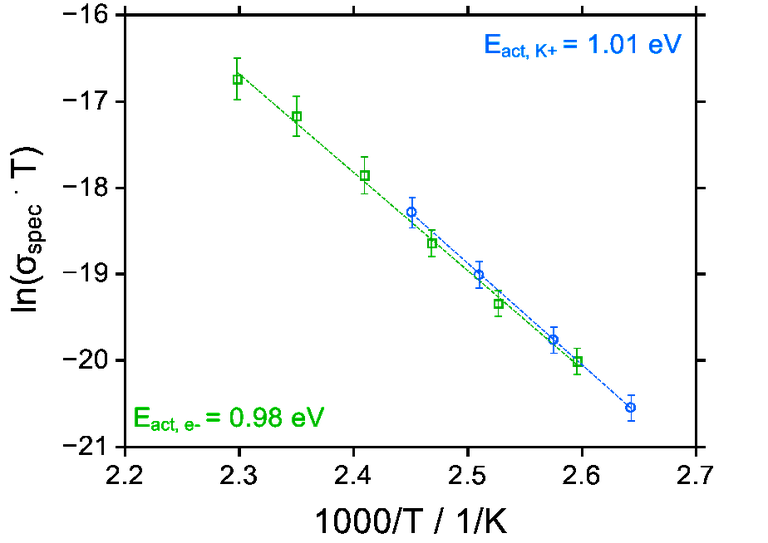
Fig. 2 Arrhenius plot of absolute ionic conductivities from e-CAIT and from K+-CAIT [2].
This left the question, why this approach worked out in the first place. Conventionally, the transport of cations towards the negatively charged front side would be expected to lead to ion blocking at the backside immediately. Here systematic long term experiments provided evidence that ion blocking only evolved on the scale of hours after considerable charge transported. As a matter of fact the evolution of an ion blocking zone was demonstrated to involve the transient dielectric breakdown of the material [3].
References
[1] J. Martin, S. Mehrwald, M. Schäfer, T. Kramer, C. Jooss, K.-M. Weitzel
Transport of ions in a mixed Na+/K+ ion conducting glass - electrodiffusion profiles and electrochemical interphase formation
Electrochimica Acta, 191, 616–623 (2016)
http://dx.doi.org/10.1016/j.electacta.2016.01.061
[2] A. Hein, M. Schäfer, K.-M. Weitzel,
Electron attachment induced ion transport — Part I: Conductivities and activation energies,
Solid State Ionics, 339, 114996, (2019)
[3] A. Hein, M. Schäfer, K.-M. Weitzel,
Electron attachment induced ion transport – Part II: The evolution of blocking of charge transport
Solid State Ionics, 339, 114997, (2019)