Main Content
Foreign ion – CAIT studies of conductivity
There are two basic versions of ion-CAIT, i. the native ion CAIT and ii. the foreign ion CAIT. The foreign cation CAIT is the version where the mobile charge carrier in the sample and the one used for attachment are chemically different.
In our first demonstration of the foreign ion CAIT we studied the Na+ conductivity of a sodium-calcium-phosphate glass by means of K+ ion attachment. Charging the surface by attachment of K+ ions induces transport of the native Na+ ions away from the surface. This, however, enforces the K+ to “follow” the Na+ ions into the material, giving rise to a concentration depth profile.

Fig. 1 Scheme of the K+@Ca30Na CAIT experiment.
Interestingly, the absolute ionic conductivities observed turned out to be slightly smaller than the ones observed in impedance spectroscopy of a fresh sample, see Fig. 2. The activation energy was basically identical, Eact (BIIT) = 0.99 eV ± 0.01 eV, versus Eact (IS) = 0.98 eV ± 0.02 eV [1].

Fig. 2 Arrhenius plot of conductivities observed in the K+@Ca30Na CAIT experiment in comparison with impedance spectroscopy data. Taken from [1].
The difference in the absolute conductivities is easily explained by the help of concentration depth profiles obtained from ToF-SIMS measurements. Such profiles are shown in Fig. 3. Evidently the native Na+ ions have been depleted and replaced by K+ ions. This constitutes a resistive layer leading to the overall decreased conductivity.

Fig. 3 Concentration depth profiles for K and Na after K+@Ca30Na CAIT. Symbols experimental data, lines: result of NPP theory. Taken from Rossrucker et al. (2012)
The pivotal result of the NPP analysis is that the diffusion coefficient of the native Na+ ions significantly depends on the local concentration; the diffusion coefficient of the K+ is effectively constant. In the original paper this was modelled heuristically. By now, we can quantitatively explain the concentration dependence of the diffusion coefficient by taking into account the populated site energy distribution of Na+ in the potential energy landscape of the glass [2].
The power of the foreign cation CAIT approach becomes evident in a study of mixed Na+/K+ conduction in a sodium and potassium containing borosilicate glass. That glass has been studied in a Cs+ attachment experiment. Again, the two native, mobile ions, Na+ and K+ have been depleted and replaced by the foreign ion, Cs+. Interestingly the K is accumulated above the bulk concentration directly in front of the Cs electro-diffusion profile (in direction of transport). The Na+ ion is further depleted towards the backside electrode, where it is almost exclusively deposited [3].

Fig. 4 Concentration depth profiles for Cs, Na and K after Cs+@D263T CAIT [3].
The concentration profiles shown in Figure 4 can be quantitatively modelled by Nernst-Planck-Poisson theory. Again, agreement between experiment and theory is only observed when assuming a variation of diffusion coefficients with local Na and K concentration by several orders of magnitude, see Figure 5.
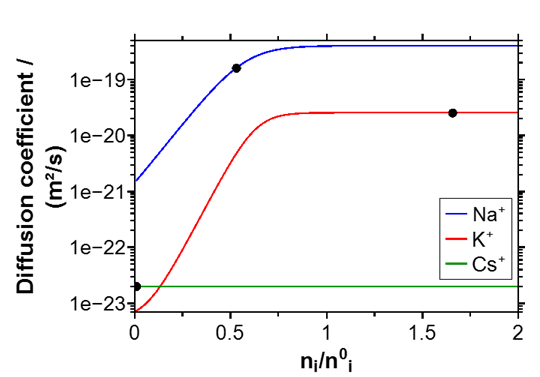
Fig. 5 Concentration dependent diffusion coefficients for native Na+ and K+ in the D263T glass. Note that D(Cs+) is effectively constant [3].
With the knowledge of ref. [2] the concentration dependence of effective diffusion coefficients can be rationalized within the concept of a concentration dependent ionic Fermi energy in the energy landscape of the glass.
Literature
[1] L. Rossrucker, P.V. Menezes, J. Zakel, M. Schäfer, B. Roling and K.-M. Weitzel
Bombardment induced potassium ion transport through a sodium ion conductor: conductivities and diffusion profiles
Zeitschrift für Physikalische Chemie, 226, 341-353, (2012)
Open Access Paper: http://dx.doi.org/10.1524/zpch.2012.0215
[2] M. Schäfer, K.-M. Weitzel
Site energy distributions of ions in the potential energy landscape of
amorphous solids
Materials Today Physics, 5, 12-19, (2018)
http://dx.doi.org/10.1016/j.mtphys.2018.05.002
[3] J. Martin, S. Mehrwald, M. Schäfer, T. Kramer, C. Jooss, K.-M. Weitzel
Transport of ions in a mixed Na+/K+ ion conducting glass - electrodiffusion profiles and electrochemical interphase formation
Electrochimica Acta, 191, 616–623 (2016)
http://dx.doi.org/10.1016/j.electacta.2016.01.061