Main Content
Mechanism and maturation of bacterial tungtoenzymes
We have identified a bacterial aldehyde oxidoreductase dependent on the presence of a tungsten cofactor (WCo). Together with some previously known similar enzymes from hyperthermophilic Archaea, these are the only known enzymes capable of reducing non-activated acids to the corresponding aldehydes, a highly interesting reaction for biotechnological applications. We are working on solving the structure of this enzyme and developing a biotechnical system of acid reduction. Another interesting aspect of bacterial WCo-dependent enzymes is their co-occurrence with MoCo-dependent ones, necessitating specific maturation pathways for either enzyme class. Based on the known genome, we are working on identifying the discriminatory reactions and on identifying the yet-unknown pathway of WCo maturation in this enzyme class.
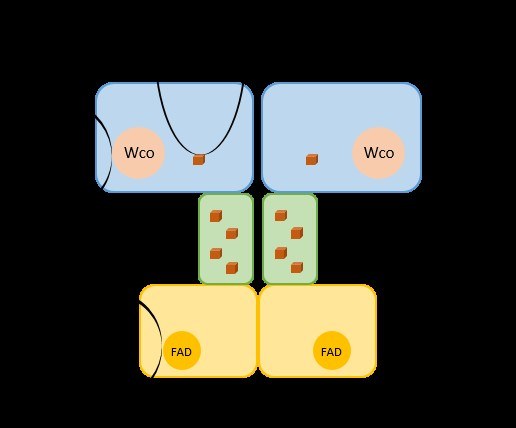
Fig. 5: Model of bacterial aldehyde oxidoreductase. The enzyme contains a W-cofactor (Wco) in its active site and additionally carries 5 Fe4S4 clusters and an FAD per protomer, which transfer electrons either to artificial viologen dyes or to NAD. Arndt F., Schmitt G., Winiarska A., Saf M.t, Seubert A., Kahnt J., Heider J. (2019) Characterisation of an aldehyde oxidoreductase from the mesophilic bacterium Aromatoleum aromaticum, a member of a new subfamily of tungsten-containing enzymes. Front. Microbiol. 10, 71