Main Content
Novel ATP-dependent carboxylases
Degradation of acetone other ketones (e.g. butanone, acetophenone or propiophenone) is initiated by the action of a novel class of carboxylases, which are proposed to activate both the ketones and bicarbonate to phosphorylated intermediates, which are then combined to the carboxylated products. The structures of acetone carboxylase and acetophenone carboxylase have recently been solved, opening the way for a more detailed analysis into their respective mechanisms.
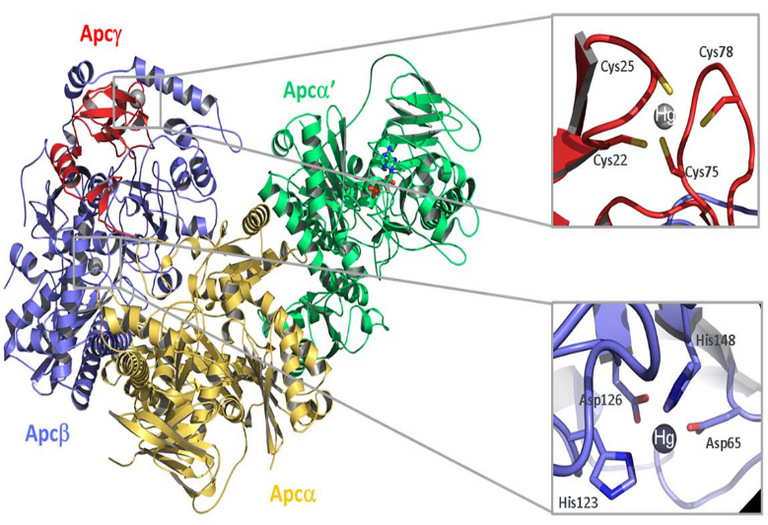
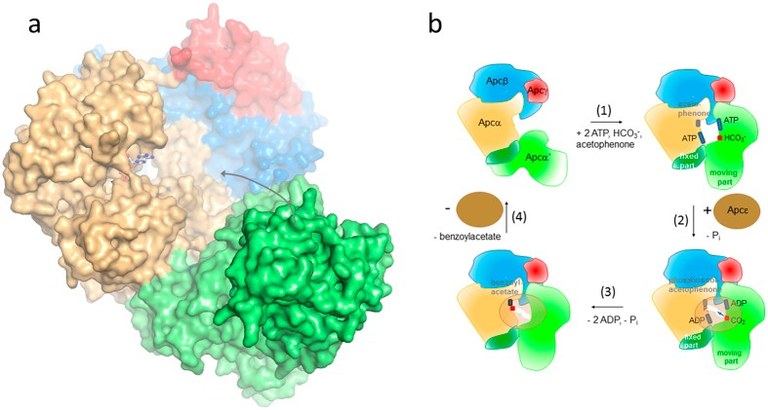
Fig. 4: left: structure of a aa’bg protomer of acetophenone carboxylase core enzyme with the two metal-binding sites highlighted. Note that the physiologically bound metal is Zn, which has been replaced by Hg in the crystals. Right: proposed mechanism, which also includes the additional subunit ApcE, which protects the activated intermediates from hydrolysis and provides a diffusion pathway from the ATPase sites to the active site of carboxylation. © Weidenweber S., Schühle K., Demmer U., Warkentin E., Ermler U. & Heider J. (2017) Structure of the acetophenone carboxylase core complex: prototype of a new class of ATP-dependent carboxylases/hydrolases. Nature Sci. Rep. 7, 39674