Main Content
Molecular Plant Physiology and Development
(Forreiter/Galland Lab)
Sessile organisms grow towards or away from an environmental stimulus like light or gravity by an auxin mediated bending. We are interested to understand the mode of action of early signalling events either initiated by light or by gravity, which ultimately lead to a gravitropic and/or phototropic growth.
So far we have succeeded in identifying two antagonistically working proteins of the signalling chain in Arabidopsis, called EHB1 and AGD12 (Fig. 1).
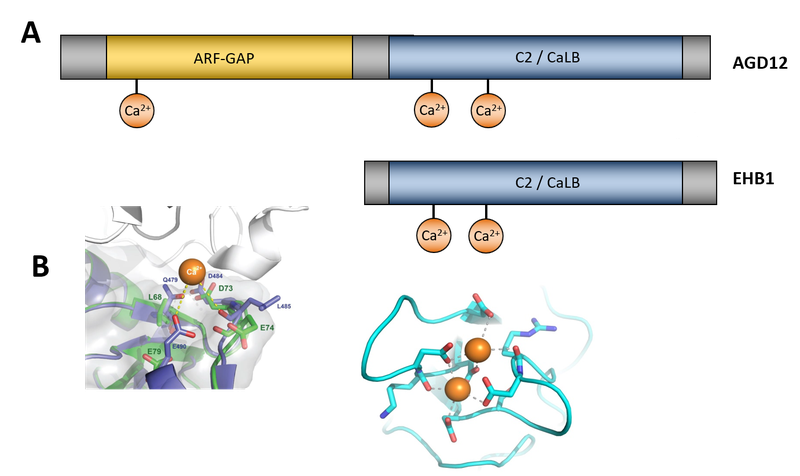
Fig.1: Comparison of domain structure of EHB1 and AGD12. (A) While both proteins contain C-terminally a fully-fledged C2 or CaLB domain, AGD12 possesses an N-terminal functional active ARF-GAP domain. (B) Right hand side: Model of the Calcium binding domain within the C2 / CaLB domain of both proteins. Left hand side: ADG12 contains an additional Ca-binding element within the ARF-GAP domain, which is absent in a human homologue, providing an additional on-off element.
EHB1 stands for “enhanced bending” (Knauer et al., Plant Physiol. 2011), since an Arabidopsis loss-of-function line reacts more strongly to a given gravity or light stimulus as the wild type, while AGD12 loss-of-function mutants (Dümmer et al., J Plant Physiol. 2016) behave exact the opposite way (Fig. 2).
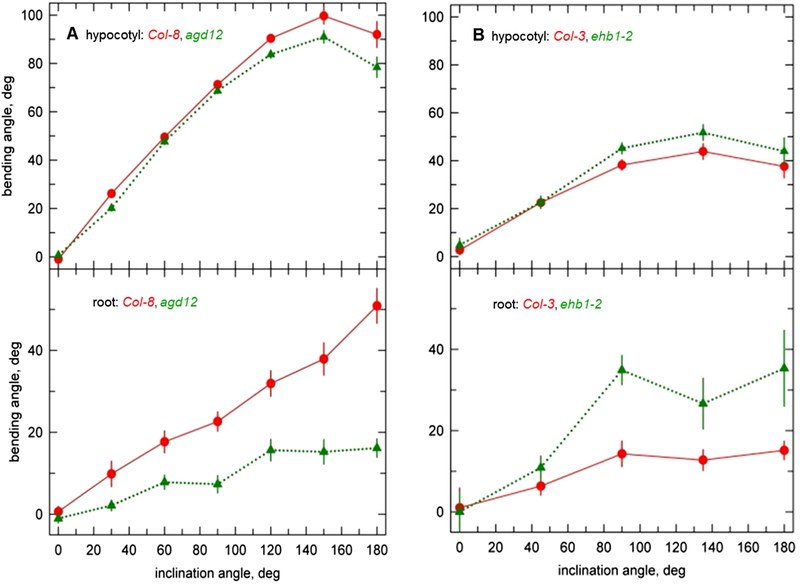
Fig. 2: The bending response obtained in stem and roots after a gravitropic stimulus (90° tilt) of wildtype (red lines) and agd12 loss-of-function mutants (green lines on the left-hand side) were compared to bending of ehb1 loss-of-function lines (green lines on the right-hand side). The results revealed an enhanced bending for ehb1 mutants, while agd12 seedling revealed lower bending angles.
Thus, both proteins might act as a regulating element during bending, were AGD12 is the effector and EHB1 serves as an inhibitor, respectively. Interestingly EHB1 gets redistributed after a gravitropic stimulus (90° tilt), while AGD12 is not affected (Fig. 3). AGD12 looks quite similar to EHB1, since both proteins contain a calcium lipid binding domain (CaLB/C2), but AGD12 additionally contains an ARF-GAP domain, which functionally links the protein pair to small G proteins of the ARF family, which play an important role in vesicle sorting in the Trans-Golgi network.
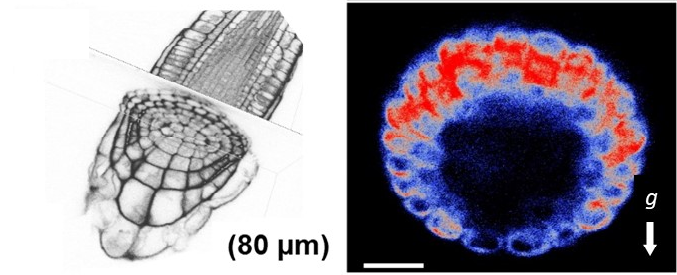
Fig. 3: After a given gravitropic stimulus, EHB1, which is under vertical growth conditions equally distributed in a ring like pattern within the root tip, shifts to the upper part of the root (left side; red: high amount of EHB1, blue: low amount of EHB1). Currently we hypothesize that the asymmetrically EHB1 distribution is one important component that contributes to the shift of Ca2+-ions to the bottom of the root, which is a well-documented event during gravity induced bending.
In a project currently funded by the DLR (Deutsches Zentrum für Luft- und Raumfahrt), we want to investigate and understand the functionality of the effector pair AGD12 and EHB1 in root tissue and at the cellular level. The aim is to investigate the redistribution of the inhibitor EHB1 in root tissue under hypo- and hypergravity conditions by fluorescence analysis using transgenic EHB1-GFP and AGD12-GFP expressing Arabidopsis mutant lines. Since both proteins contain a calcium-binding domain, both transgenic lines were additionally supplied with a calcium sensing reporter protein (R-GECO1; Waadt et al., New Phyt. 2017). In Fig. 4 GFP-fluorescence and RFP-fluorescence were depicted. While GFP-fluorescence indicated the spatial distribution of EHB1 in root tissue, RFP-fluorescence indicated Ca2+ distribution within the root tip.
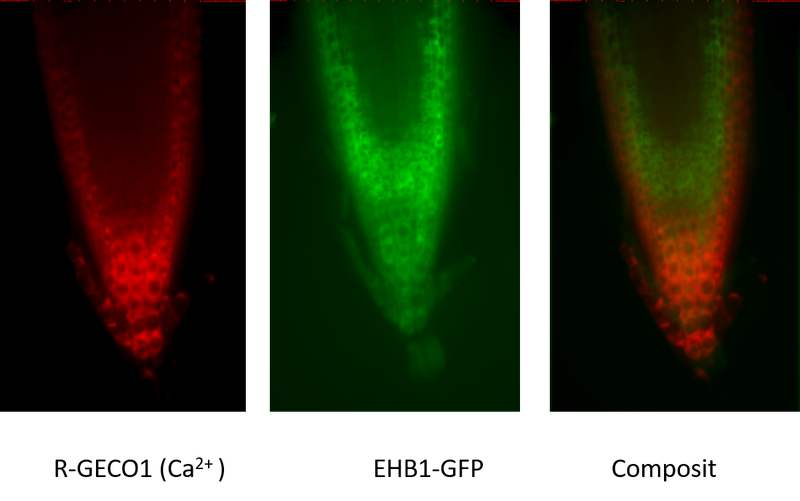
Fig. 4: Comparison of calcium induced red-fluorescence (left image) and EHB1 (GFP-fluorescence; central image) revealed a different spatial distribution within the root tip.
Being part of the 36. DLR parabolic campaign in September 2019 (Fig. 5), we successfully analysed wild type Arabidopsis ehb1 loss-of-function mutants as well as EHB1 overexpressing seedlings under hyper-g and µg conditions. Seedlings were investigated by confocal laser scanning microscopy using FLUMIAS, a spinning disc microscope developed by AIRBUS for live cell imaging under zero g conditions.

Fig. 5: Scenes from the 36. DLR parabolic flight campaign in September 2019. During parabolic flights scientist can operate for 22 seconds under almost zero-G conditions. After a steep climbing with 1.8 g, were the plane almost stalls, it enters zero-g conditions at the 1st inflection point of the parabola (top left). Weightlessness will last until the 2nd inflection. During that time Ca2+ -distribution was analysed using the FUMIAS microscope. Top right: Michaela Dümmer (AG Forreiter), Peter Cordes, Detlef Wilde (both AIRBUS SD) and Marjorie Guichard (AG Grossmann) operating FLUMIAS on the first flight day. On the second flight day (bottom left) we received support from Matthias Maurer, the designated successor of Alexander Gerst, who was quite interested in our experiments, since farming of plants in space to supply further mission sustainable with oxygen and nutrition, requires detailed knowledge of how plants develop under microgravity. Bottom right: Ready to launch.
We took advantage of a “RootChip” supply system during flight, which was developed by Guido Grossman and coworkers in Heidelberg (Grossmann et al., Plant Cell 2011). It could be shown so far that after a given gravitropic stimulus microgravity induced a redistribution of Calcium-ions from the lower part of the plant toward the upper regions, while in EHB1 function mutants remain unaffected.

AG Forreiter (2020), from right to left: Ruben Fritsche (MSc-Student), Dr. Magnus Rath (Postdoc), Dr. Michaela Dümmer (Postdoc), Prof. Dr. Christoph Forreiter (AG-Leitung), Christian Wenzel-Benner (IT-Beratung, embedded systems).