Main Content
Aims and methods for the DEAPMARK study
The DEAPMARK study is in cooperation with the Kilimanjaro Christian-Medical Center and -Research Institute (KCMC, KCRI) (Fig. 4). Provided the local settings, the pretest study is required to ensure feasibility and uncompromised biomaterial quality, suitable for future high throughput technologies. Our preliminary work will be consolidated and used to standardize protocols for all aspects of the study, all the way from recruitment to biosample collection, preparation, transportation and ultimate long term storage in the KCRI biobank.
Quality control of biosamples, biomarkers to be tested and transportation to our institute will be also assessed by means of proteomic approaches and participation of local labs in external quality assessment programs (EQAs). Subsequently, we will investigate pathobiochemical and immunological mechanisms underlying the development of NCDs in a developing country. Direct comparison with our data from national and European multicenter studies places us in the exclusive position of being able to identify epidemiological and trigger factors for development of NCDs in our society.
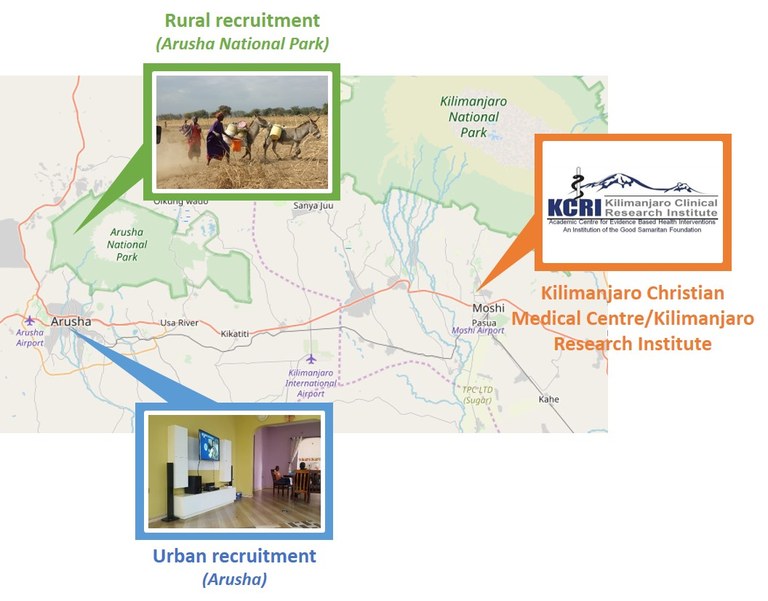
Figure 4: Recruitment sites and cooperation partners for the DEAPMARK study.