Main Content
Molecular Mechanisms of immune cell dynamics and plasticity
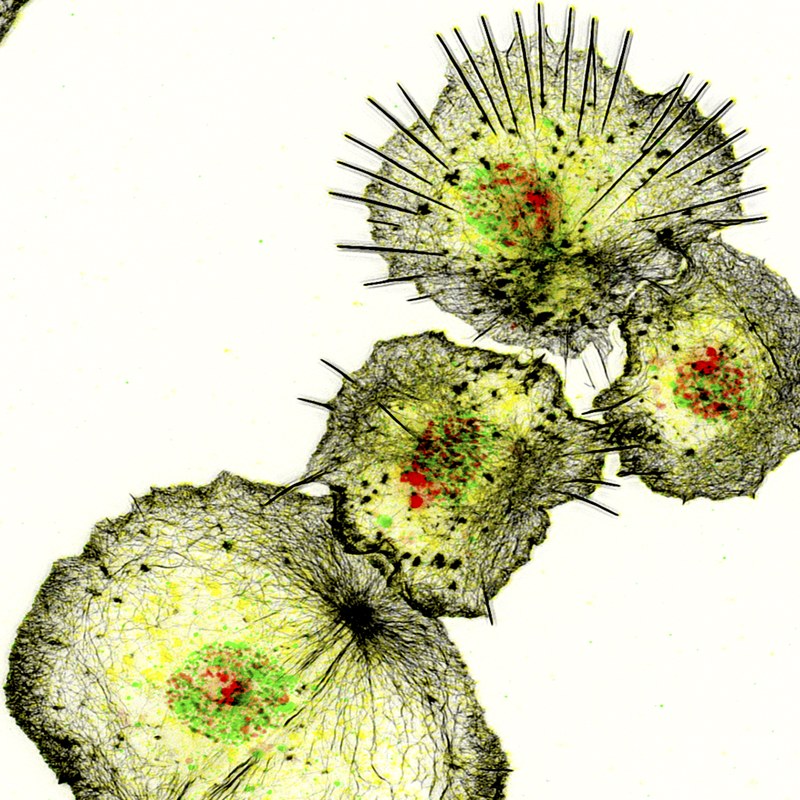
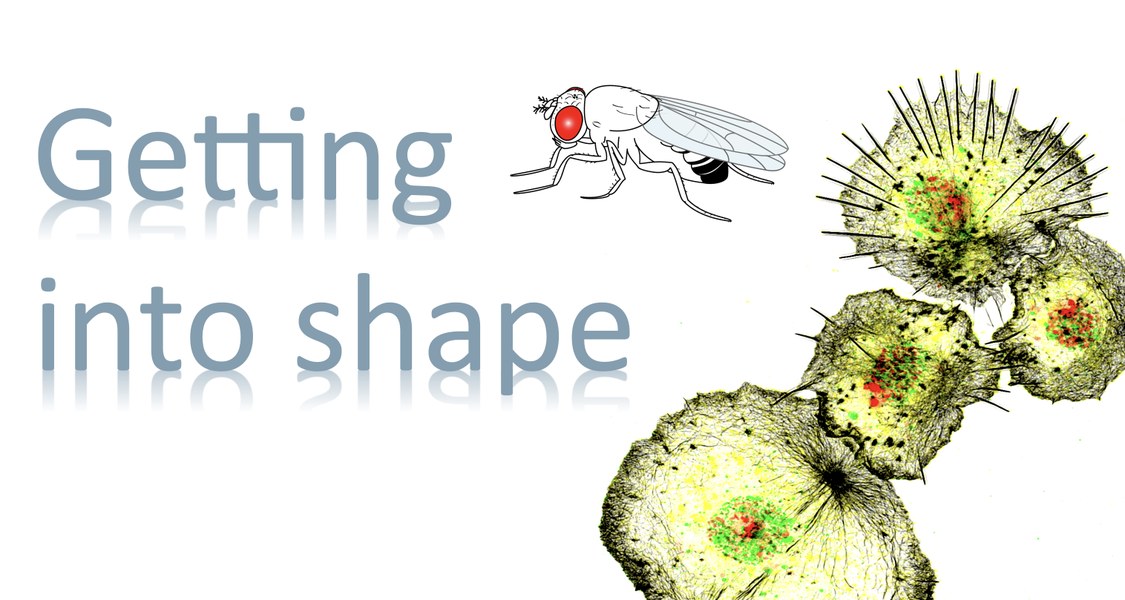
Foto: S. Bogdan The innate immune system relies on a variety of conserved cellular and molecular strategies to mediate pathogen defense, tissue remodeling and repair. Drosophila is a suitable genetic model organism for studying blood cell development and innate immunity. Similar to mammals, the first line of defense against invading pathogens and wounds in Drosophila relies on both a humoral response, in which effector molecules such as antimicrobial peptides are secreted into the hemolymph, and a cellular response, in which pathogens are phagocytosed by blood cells, particularly macrophages. We have established Drosophila macrophages as an excellent model system that combines many advantages of cultured cells with an in vivo genetic model system to study conserved gene functions in single cell migration and immune cell response to injury. Using various RNAi and overexpression approaches, we have already identified numerous cytoskeletal and signaling molecules that control immune cell morphology and motility. Recently, we started to combine single-cell transcriptomics and high-resolution microscopy to further investigate the heterogeneity and plasticity of Drosophila blood cells. We identified undifferentiated and specified hemocytes with distinct molecular signatures associated with different functions such as antimicrobial, antifungal immune defense, cell adhesion or secretion. Our data represent the first molecular description of Drosophila pupal blood cells and provide first insights into the functional diversification and plasticity of blood cells during development.
You can read more about this topic in the press release of the University of Marburg or listen our podcast "cells in motion".Molecular mechanisms and players in calcium-dependent epidermal wound healing
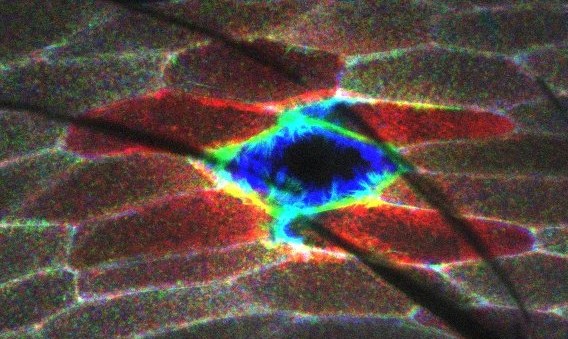
Epithelia are continuous layers of polarized cells that form selective barriers lining most of our organs, including the epidermis of our skin. When injured, epithelial tissue must rapidly seal and remodel the injured area to prevent infection and maintain tissue homeostasis and function. Drosophila has proven to be a valuable genetic model system for studying wound healing of a simple epithelium in tissue injury. Calcium is a known ubiquitous secondary messenger and the first “damage signal” after cellular and multicellular injury. However, it is still unclear how wound-induced calcium signaling is initiated and how calcium promotes rapid epithelial wound closure and immune responses in vivo. Previous studies suggested that mechanically gated calcium channels initiate calcium influx, while downstream calcium-responsive effector proteins such as EF-hand-containing cytoskeletal proteins like Swip-1 convert the damage signal into morphological changes driven by actin cytoskeletal reorganization. We use our recently established Drosophila single-cell wounding model system to combine high-resolution live cell microscopy and genetics to test this hypothesis and define the conserved molecular events and players that drive epidermal wound closure and how immune cells contribute to wound healing. Specifically, we will identify and characterize the conserved genes required for the initiation and propagation of calcium waves in injured epidermal tissue. Second, we will identify and characterize the genes encoding calcium-regulated effector proteins that induce changes in cell morphology, motility and immune cell responses. Finally, we will functionally analyze the role of immune cells in epidermal wounds.
You can read more about this topic in the press release of the University of Marburg.Molecular mechanisms controlling invasive collective cell migration
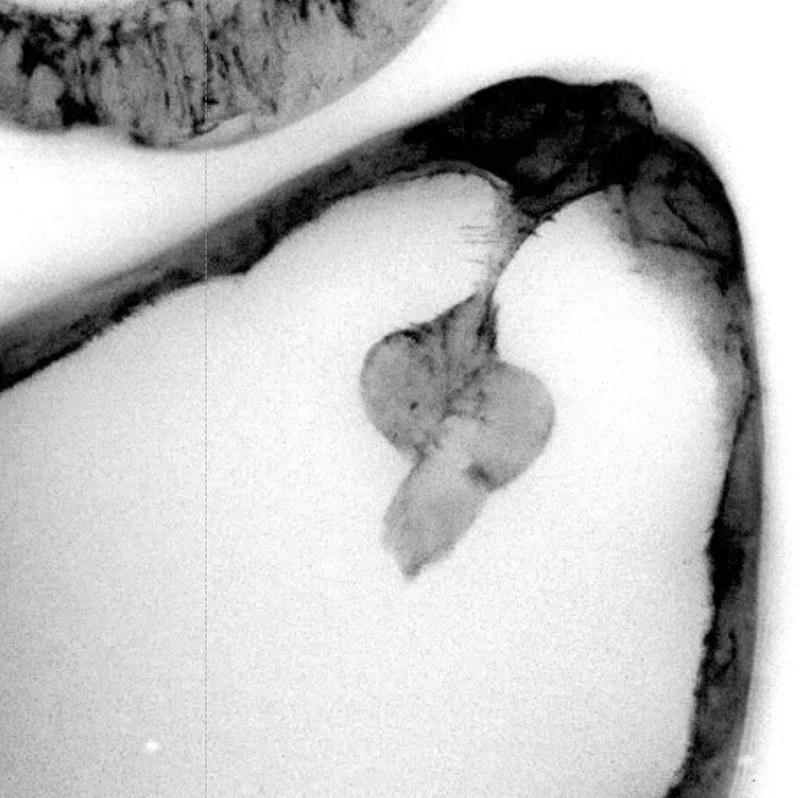
Foto: S. Bogdan During invasive collective cell migration, a group of cells must exert force to penetrate through the ECM or other cells. The Drosophila ovary is an excellent model system to study the molecular basis of cell-cell communication during collective cell migration and cell invasion. In the ninth developmental stage, a group of 4-8 follicle cells detach from the anterior follicular epithelium (delamination). These so-called border cells migrate posteriorly as a cell cluster between the nurse cells until they reach the oocyte. The delamination and invasive migration behavior of border cells serves as a simple genetically accessible in vivo model for the metastasis of epithelial tumors that underlie conserved signal transduction cascades. Thus, transformation of follicular epithelial cells into invasive cells is mediated by the JAK/STAT signaling pathway, which activates a key C/EBP transcription factor Slow border cells (Slbo). Similarly, activation of JAK/STAT signal transduction and induction of C/EBP is associated with metastasis of numerous tumors in humans.
See also our video.Calcium-dependent cell remodeling in cardiac physiology and pathophysiology
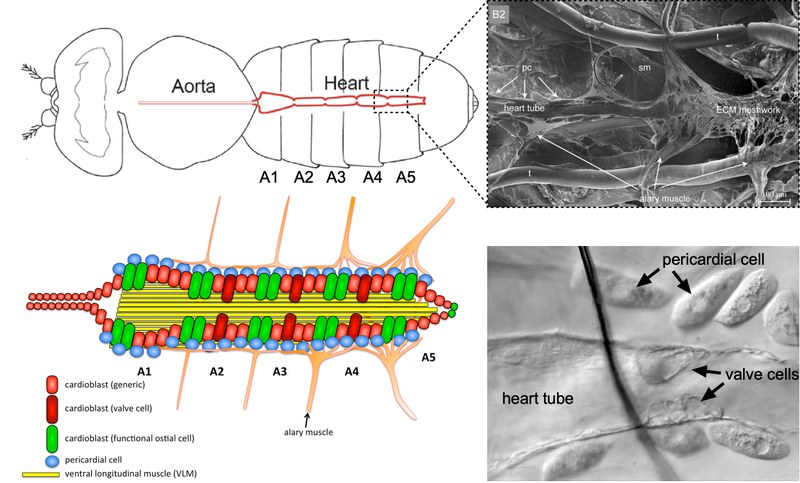
Foto: S. Bogdan Heart disease is still the most common cause of death worldwide. The heart responds to pathological stress states such as hypertension or ischemia with myocardial hypertrophy, fibrosis and scarring. This maladaptive response is characterized by a pronounced wound healing and remodeling process that leads to altered physiological function and the development of pathological dysfunction and heart failure. At the cellular level, cardiac remodeling is accompanied not only by altered expression and regulation of ion channels and transporters that cause arrhythmias, but also by dramatic changes in cell size and shape due to reorganization of the actin cytoskeleton. Enlarged hypertrophic cardiomyocytes undergo a marked transformation from a round cell shape to a flat and expanded morphology with characteristic changes in the organization of the sarcomere filaments. These structural changes are at least partially calcium-dependent. The underlying molecular mechanisms of how cardiomyocytes reorganize their cell shape and behavior are still poorly understood. To better understand these mechanisms, we have started to establish the Drosophila heart as a genetic model system. In this research project we want to use different animal models (mouse rat and fly) in cooperation with other research groups to decipher the conserved function of calcium-dependent actin regulators (e.g. Swip-1) in the physiology and pathophysiology of the heart (cardiac remodeling and aging). Our project aims to identify evolutionarily ancient strategies of wound healing as potential key processes to trigger cardiac remodeling processes and thereby identify new potential treatment targets.
You can read more about this topic in the press release of the University of Marburg.