Main Content
The scientific background to the clinical research group 325
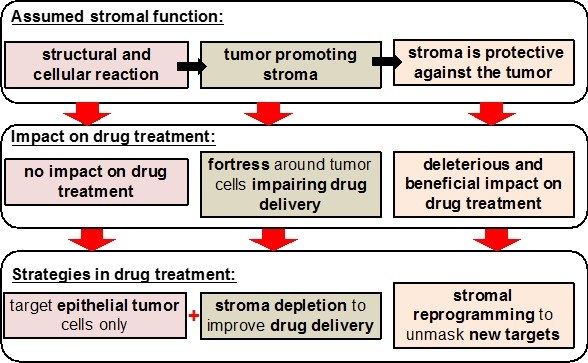
Despite significant research efforts in the last decades, pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal cancer types known with an extremely poor prognosis. One hallmark of PDAC is its desmoplastic nature characterized by a high content of extracellular material deposition and the presence of numerous stromal cells, including activated fibroblasts/stellate cells and various immune cells. The contribution of stromal elements to the etiology of PDAC is complex and has undergone changing views in the recent past. However, it is clear that stromal cells are major determinants of the biology of pancreatic cancer. As such, they represent central players regulating the outspoken aggressiveness of PDAC with its early propensity for metastasis, its high degree of chemoresistance and the significant local host immune suppression.
In Clinical Research Unit 325 (CRU325/KFO325), we seek to shed light on these central features of the disease and elucidate underlying pathological mechanisms involved in the crosstalk of pancreatic tumor and stroma compartments. Our aim is to develop translatable approaches to target different stromal/immune cell populations and their corresponding signaling systems in order to combat the clinical failures associated with previous therapy regimes mainly focusing on the cancer cell compartment. Furthermore, we want to identify microenvironment-related and hitherto unknown PDAC biomarkers, allowing for a future rational design of novel diagnostic and/or therapeutic approaches.
Specifically, we will focus on the pathological roles of T cells, NK cells, macrophages, neutrophils and fibroblasts, using a sophisticated array of well standardized patient material, mouse models and in vitro systems. All patient material will be characterized by next generation sequencing including transcriptomics and PDAC gene mutation analysis, and by immune phenotyping and expert histological assessment. Ex vivo cell isolations as well as animal handling will be carried out centrally to ensure the highest comparability of data obtained in the different CRU projects, thereby maximizing the scientific output. These activities will be part of a larger central project platform, which is run by experts in their fields and which comprises patient sample biobanking, in vivo imaging and medicinal chemistry. All principal investigators participating in this CRU were selected based on their scientific excellence and their conceptual fit, integrating numerous clinical and basic research specialists into a synergistic translational network on stroma targeting and reprogramming with the ultimate goal of improving the long-term perspectives of PDAC patients. In addition, the CRU will structure and integrate research on tumor-microenvironment interactions in PDAC at the Marburg medical faculty and will provide possibilities for career development of young researchers and clinician scientists.