Main Content
CK2 functions in hematopoiesis
Jonathan Lenz, Marie Unverzagt
Hematopoietic differentiation is driven by changes in the transcriptome and subsequently the proteome of progenitor cells. During development these changes occur gradually over extended time frames. However, some stimulus-induced differentiation events occur in a very short time window. One such event is the production of specialized immune cells (lamellocytes) from hemocyte progenitor populations upon infection of Drosophila larvae. We are trying to understand how this rapid differentiation is controlled on the level of gene expression.
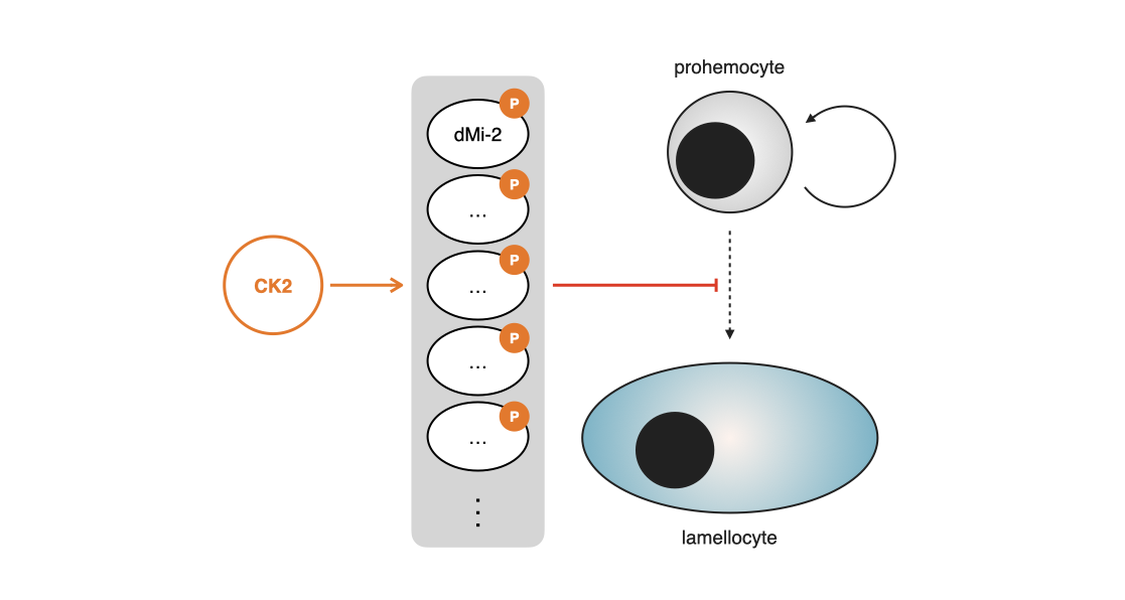
Our previous studies have identified the chromatin remodeling complex dMi-2/dNuRD as a key player in repressing this differentiation event (Lenz et al., 2021). Recent observations hint to an involvement of the CK2 kinase in this process: A loss-of-function mutation in the calatytic alpha-subunit of casein kinase 2 (CK2α) leads to increased commitment of hemocytes to the lamellocyte lineage (Hirschhäuser et al., 2021). Interestingly, we identified dMi-2 as an in vitro target of CK2 many years ago (Bouazoune et al., 2005), making it a potential transducer of CK2-mediated functions to chromatin.
In this project we are addressing, which substrates of CK2 might be responsible for the hematopoietic phenotype, focusing on dMi-2 in particular. For that we use a cell culture model: S2 cells, which resemble embryonic hemocyte progenitors. Upon RNAi-mediated depletion of CK2α we investigate changes in the S2 cell phospho-proteome as well as their transcriptome. This will serve as a reference point in order to ultimately investigate CK2-mediated phorphorylation events during hematopoiesis in vivo.
This project is funded by the Deutsche Forschungsgemeinschaft (DFG, grant BR2102/9).