Main Content
Adhikary Lab
Gene Regulation
Deregulated transcription is causal for a large fraction of human diseases such as cancer and autoimmune disorders. Our interest is to elucidate how regulation of specific transcripts relevant to pathogenesis is brought about mechanistically. We use assays of upstream signaling events and signal integration at model gene loci, genetic approaches, and pharmacological intervention as well as genomic, proteomic, and lipidomic high-throughput approaches to characterise regulatory events in primary cells from human donors and in model cell lines. In particular, we focus on:
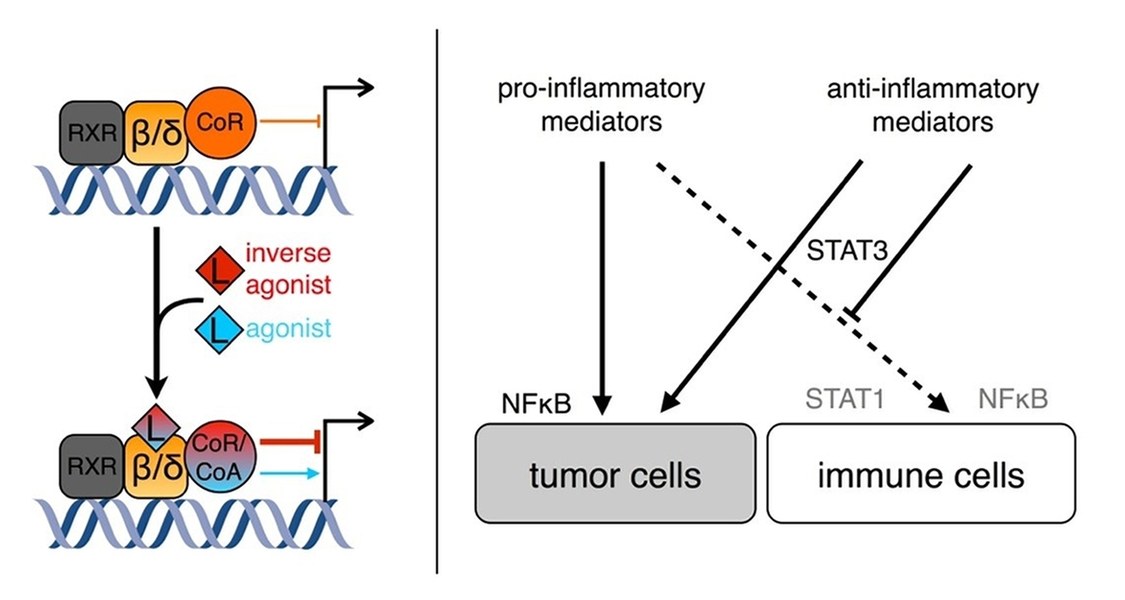
1. The mechanism of transcriptional repression by novel synthetic inverse agonists of the nuclear receptor PPARbeta/delta (peroxisome proliferator activated receptor beta/delta).
The nuclear receptor superfamily is a group of evolutionary related DNA-binding soluble proteins. DNA-bound NR complexes regulate target gene expression in a ligand-dependent fashion, making them useful targets for pharmacological regulation of distinct gene sets.
Inverse agonists are negative ligands which, in contrast to agonists, actively downregulate transcription of a subset of direct target genes of the respective receptor. PPARbeta/delta is a ubiquitously expressed nuclear receptor which regulates the expression of genes involved in processes such as wound healing and inflammation, fatty acid metabolism, and tumorigenesis. PPARbeta/delta inverse agonists, which were developed by the group of Wibke Diederich, downregulate expression of a subset of target genes in a dominant fashion. The project aims to identify the factors mediating inverse agonist-dependent repression and to characterise their mechanism of action.
2. Analysing how tumors influence their microenvironment by means of soluble mediators in order to evade pro-inflammatory activation of stromal immune cells, especially macrophages, in ovarian carcinoma and other tumor entities.
Tumors frequently evade immune surveillance by evoking a wound healing program which locks immune cells in the tumor microenvironment in a non-inflammatory resolution state. Ovarian cancer is accompanied by accumulation of a malignant effusion in the peritoneal cavity called ascites that constitutes the tumor microenvironment. Ascites harbours tumor and stromal cells, contains a plethora of soluble mediators, and promotes dissemination. We aim to identify factors which are necessary and sufficient to suppress transcription of key pro-inflammatory genes as well as their mechanisms of action in macrophages by screening candidates derived from transcriptomic, proteomic, and lipidomic analyses.
Last update: 4.11.2022