Main Content
PDAC microenvironment: reciprocal cross-talk between Tc17 cells and cancer associated fibroblasts
Funding: DFG KFO325, HU 1824/5-2
Pancreatic cancer (Pancreatic Ductal Adenocarcinoma, PDAC) is characterized by highly desmoplastic stroma consisting of fibroblasts with an activated phenotype that associates with a poor prognosis. In our new manuscript, we show that increased abundance of Tc17 cells was highly significantly associated with reduced survival and advanced tumor stage in PDAC based on analysis of tissue microarrays from 107 patients. Co-culture experiments in vitro and mouse models in vivo revealed that Tc17 cells promoted differentiation of inflammatory cancer associated fibroblasts (iCAFs) in an IL-17A/F and IL-17RA dependent manner. In turn, Tc17 induced iCAFs accelerated the growth of mouse and human tumors. Thus, we identified Tc17 as a novel pro-tumorigenic CD8+ T-cell subtype in PDAC and described a crosstalk between three cell types, Tc17, fibroblasts, and tumor cells, that promote PDAC progression and result in a poor prognosis for patients. Our next projects address characterization, understanding the origin and sensitivity to the induction of exhaustion (induced fatigue?) of Tc17 cells in PDAC. We plan to characterize intratumoral Tc17 cells including TCR specificity and transcriptional profile, and accompanying changes in tumor cells obtained from human PDAC patients by single‐cell RNA sequencing. Our aim is to understand Tc17-driven changes in tumor cells, in order to design new therapy strategies.
If Tc17 cells are induced in tumor draining lymph nodes, then circulating Tc17 cells should be detectable in peripheral blood (PB). To prove this hypothesis, we plan to carry out spectral analysis of PB from PDAC versus healthy control (HC) individuals using a 40-marker panel. We believe, that this analysis will detect a specific Tc17 subpopulation in PB, which could serve as a bio-marker of PDAC outcome.
Considering that Th2 and Treg cells are also involved in PDAC our long-standing goal is to understand T-cell specific trajectories driving pancreatic cancer progression.
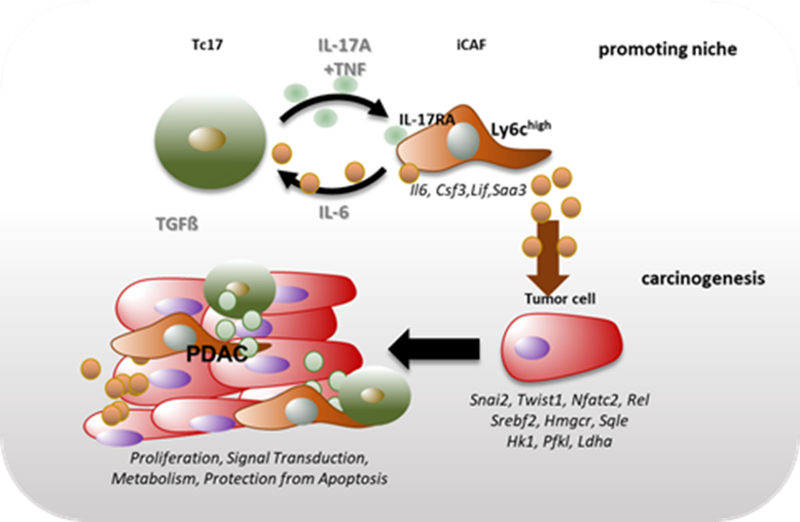
Figure 1. Proposed mechanism of an indirect cancer-promoting role of Tc17 cells in PDAC. Tc17 cells via synergistic effect of secreted cytokines, IL-17A and TNF, shift PSC differentiation towards iCAF formation in a IL-17RA-dependent manner. In turn, Tc17-induced iCAF promote Tc17 differentiation via secreted IL-6 in combination with TGFβ. Furthermore, Tc17-induced iCAF imprint pancreatic tumor cells with a unique transcriptional profile characterized by expression of genes involved in proliferation, signal transduction, metabolism and protection from apoptosis, thereby enhancing tumor growth.