Main Content
Dissecting the role of mitochondrial complex V restriction as a checkpoint for the differentiation and function of CTL and Tc17 cells
Funding: GIF I-1474-414.13/2018
It is well established that effector CD8+ T cell function and differentiation is governed by a number of immune checkpoints and regulatory signals. An increasing body of evidence strongly indicates that like effector, naïve CD8+ T cells may be regulated by a number of intrinsic modulators giving rise to heterogeneity among individual resting cells. These data suggest that even prior to encountering an antigen, naïve T cells with identical TCR are heterogeneous and bear diverse intrinsic properties to respond and differentiate.
Our preliminary study demonstrates that mitochondrial complex V restriction, defines a novel peripheral naïve CD8+ T progenitor, named hpTn, phenotypically distinct from its descendant, dpTn, by reduced OXPHOS, substantially elevated mitochondrial membrane potential, and lower propensity to respond to diverse stimuli. Upon in vitro activation this naïve CD8+ T progenitor showed preferential ability to differentiate into pro-inflammatory Tc17, while its mature progeny preferentially acquired the canonical cytotoxic (CTL) phenotype. Using pharmacological inhibitors to mimic complex V restriction, we illustrated that mitochondrial hyperpolarization, limits naïve CD8+ T cells propensity to respond to diverse stimuli independent of ATP production, potentially by inducing mitochondrial biogenesis arrest.
Overall, our preliminary study defines mitochondrial hyperpolarization induced by complex V restriction as a critical checkpoint controlling naïve CD8+ T cells propensity to proliferate and differentiate upon stimuli. Following these results we now propose to combine the Berger group expertise in naïve T cell biology and metabolism, with the Huber group skills in dissecting naïve T cells differentiation towards functionally distinct subpopulations to (i) uncover the role of mitochondrial complex V restriction in Tc17/CTL fate decision and resulting function, (ii) dissect the origin of the two novel Tn subsets, and (iii) find the mechanism by which mitochondrial membrane polarization controls Tn propensity to respond to stimuli.
If successful our study will demonstrate that part of the recent thymic emigrants do not proceed to maturate and maintain their reduced proliferative capacity and high activation threshold for a very long time. In addition, our study will point to mitochondrial membrane hyperpolarization mediated by complex V restriction as the mechanism governing the hypo-responsiveness of the Tn peripheral progenitor. Finally, our study will demonstrate that complex V restriction dictates Tc17 or CTL fate decision of naïve CD8+ T cells within in vivo models of EAE and Listeria infection. Thus, our findings will provide a novel strategy for "on demand" modulation of CD8+ T cell differentiation into CTLs or pro-inflammatory Tc17 cells, which can be used to enhance the efficacy of vaccines against infectious diseases, immunotherapy targeting cancer as well as treating autoimmune diseases.
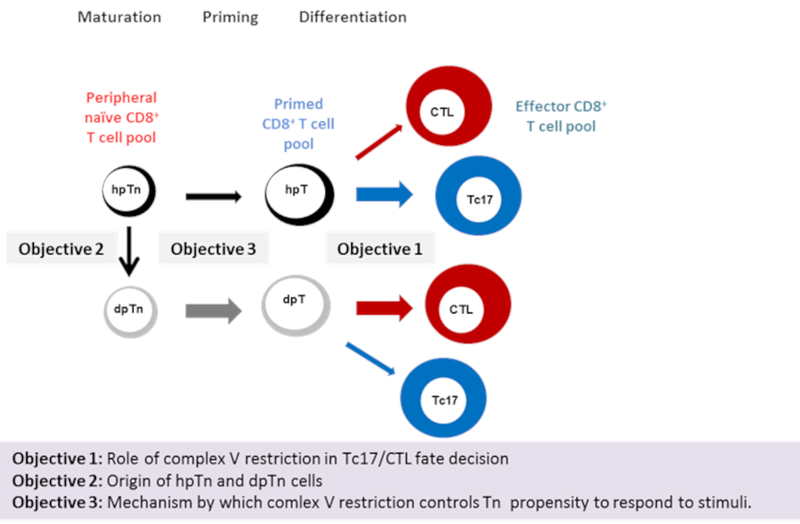
Schematic representation of the described objectives. Our preliminary study demonstrates that mitochondrial complex V restriction, defines a novel peripheral naïve CD8+ T progenitor, named hpTn, phenotypically distinct from its descendant, dpTn, by lower propensity to respond to diverse stimuli. Upon in vitro activation this Tn progenitor showed preferential ability to differentiate into pro-inflammatory Tc17, while its mature progeny preferentially acquired the CTL phenotype.
Our objectives are:
1. Uncover the role of mitochondrial complex V restriction in Tc17/CTL fate decision and resulting function in infection and autoimmunity.
2. Dissecting the origin of the two novel Tn subsets.
3. Exploring the mechanism by which mitochondrial membrane polarization controls Tn propensity to respond to stimuli.