Main Content
Research Group Cellular Signal Transduction
(Tumor-Stroma signaling)
-PI: Prof. Dr. Matthias Lauth-
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) exhibits the poorest prognosis of all solid tumors, with a median survival of 6 months and steadily increasing incidence rates in the industrialized world. This malignancy is projected to become the second leading cause of cancer death by 2030 in the US. Clinical diagnosis is often delayed due to the relatively unspecific clinical symptoms such as back pain, loss of appetite, loss of weight and new onset of diabetes, and thus early diagnosis is rare.
Upon diagnosis, >80% of patients have either locally advanced disease or distant metastases, mostly to the liver, that render surgical intervention impossible. 15-20% of patients can be resected with complete removal of the pancreatic tumor, however, local or distant recurrence is often observed within the first 2 years after operation. Systemic chemotherapy is the only treatment option for patients with advanced, metastatic PDAC. Most patients that present with advanced disease die within 12 months of diagnosis since many of them have chemorefractory disease, making the development of novel therapeutic approaches mandatory.
Histologically, PDAC is characterized by a dense stromal architecture with massive extracellular matrix (ECM) deposition, rendering this tumor entity as one of the most stroma-rich solid tumor types (see figure 1 for example).
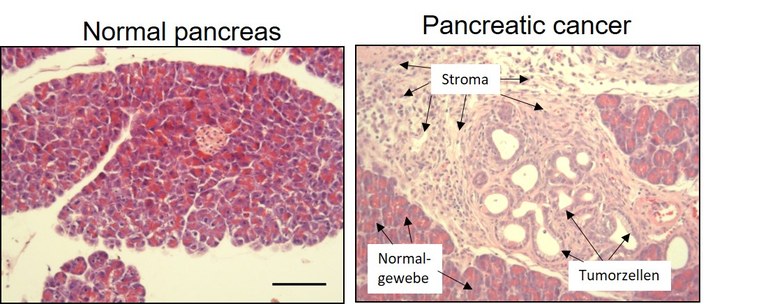
Figure 1: Tissue section of a normal pancreas (left) and of pancreatic cancer (right). Note the prominent stroma reaction in the cancer tissue.
The vast ECM deposition (Collagens, Fibronectin, Hyaluronic acid, etc.) results in a significant tissue stiffening promoting the aggressiveness of pancreatic tumors. The tumor cell-centric view of previous decades has probably contributed to the lack of significant progress in successful drug development for pancreatic cancer. It is now undisputed that the stroma is a defining feature of this disease, regulating central processes such as tumor growth, vascularization, drug responsiveness and metastasis. As such, the tumor microenvironment (TME) itself has become a target of today’s drug development efforts and is the subject of the research activities of our group.
Tumor-stroma interactions in pancreatic cancer
In pancreatic cancer, tumor cells secrete a wide variety of signaling molecules which act on surrounding normal cells of mesenchymal or immune origin (see figure 2 for illustration). One subpopulation of mesenchymal cells (the cancer-associated fibroblasts; CAFs) is for instance responsible for the outspoken desmoplastic histologic appearance of PDAC, which results in fibrosis and tissue stiffening. Other subpopulations orchestrate the interaction with cells of the immune system, leading to a significant local immune evasion observed in this disease.
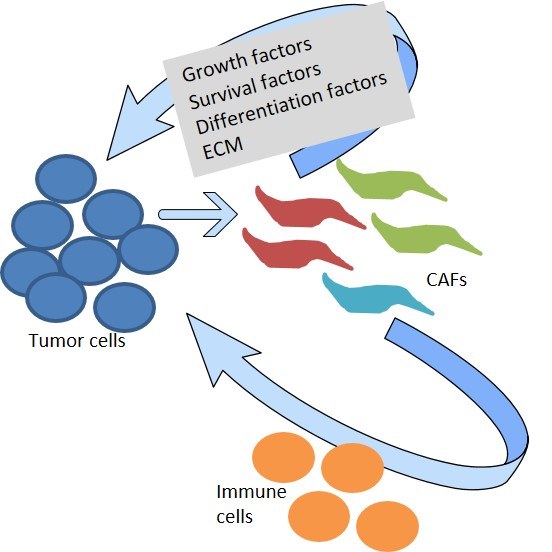
Figure 2: Schematic diagram depicting the cross-talk between tumor cells and the microenvironment. This crosstalk regulates cancer growth, chemoresistance and metastasis. CAFs = Different subpopulations of cancer-associated fibroblasts.
Our group focuses on the crosstalk between tumor cells and the microenvironment and encompasses the interaction with CAFs or cells of the immune system, such as macrophages, neutrophils or different T-cell types. Moreover, also components of the extracellular matrix interact with various cells of the tumor, influencing the overall biology and complicating our understanding. At the end of the day, what we are interested in is to discover new points of attack which can be harnessed to design novel approaches to improve the therapy of patients suffering from pancreatic cancer.
Our activities on pancreatic cancer are part of a DFG-funded consortium on the interplay between tumor and stroma compartments: Have a look at the members and the research done within the clinical research unit 325: