Main Content
Subproject 1
Human leukocyte antigen (HLA)-transgenic mice as a pre-clinical model of pemphigus vulgaris
Summary
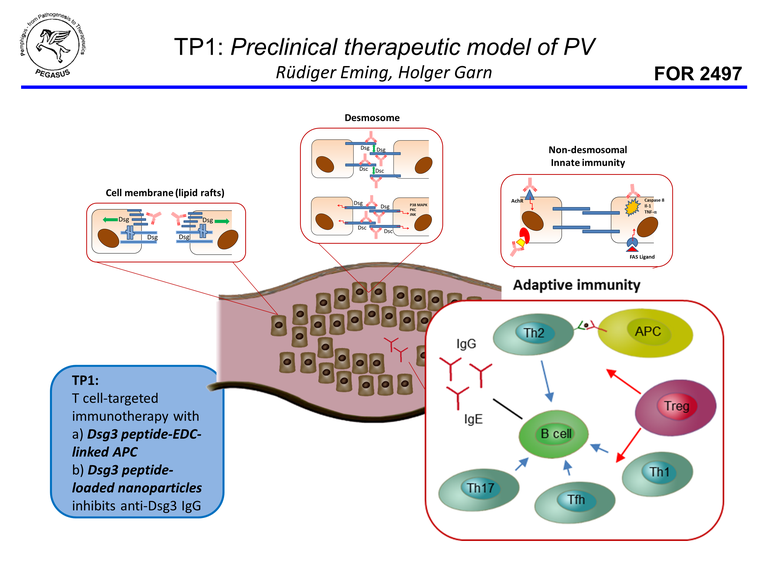
The project entitled “Human leukocyte antigen (HLA)-transgenic mice as a pre-clinical model of pemphigus vulgaris” deals with the pathogenesis of an autoantibody-mediated autoimmune disorder of the skin and the mucous membranes, called pemphigus vulgaris (PV). A transgenic mouse model expressing human genes that are strongly associated with the disease is of central importance to the project. Using this mouse model, the immune response to the major autoantigen in PV, desmoglein 3 (Dsg3), will be investigated by Dsg3-immunization. CD4+ T lymphocytes that are considered to be crucial for the initiation of the disease, will be characterized phenotypically and functionally. CD4+ T lymphocyte subpopulations will be investigated thoroughly by single cell sequencing. In a next step, these autoreactive Dsg3-specific CD4+ T cells will be targeted by various strategies such as a technique called DNAzyme. Using this method important transcription factors which are responsible for the differentiation and for the particular function of a CD4+ T cell subpopulation will be inactivated and thus a T cell activation-based initiation of the humoral immune response against Dsg3 might be prevented or reduced. Another strategy aims at converting autoreactive effector CD4+ T cells into immunoregulatory T cells finally to restore immune tolerance to Dsg3. HLA-transgenic mice will be pre-treated with 4 Dsg3-polypeptides (epitopes) bound to nanoparticles prior to Dsg3-immunization in order to prevent the formation of anti-Dsg3-IgG. Preliminary experiments demonstrate that by applying this method the immunization induced production of anti-Dsg3-IgG can be prevented. However, the efficacy of the Dsg3-peptide nanoparticle approach is going to be investigated in a “therapeutic setting” as well. Mice expressing a humanized Dsg3 protein, will be used as an additional mouse model in this project. Bei crossing both mouse lines, we would like to generate a mouse model which is humanized for the autoantigen Dsg3 and very important immune molecules (HLA, CD4). Dsg3-immunization of this novel mouse line, might break the immunological tolerance to Dsg3 and eventually lead to the manifestation of a clinical phenotype in these animals, resembling PV. The immune mechanisms that are responsible for tolerance induction and for maintaining immune tolerance to Dsg3, respectively, will be intensively investigated in the different mouse models to obtain insights into basic immunological principles.
Contact:
Philipps University Marburg
Prof. Dr. Holger Garn
Project leader TP1
Biochemical Pharmacological Center (BPC)
Karl-von-Frisch-Str. 2
D-35043 Marburg
Tel +49 (0) 6421 28 66040
Fax +49 (0) 6421 28 21637
E-Mail: garn@staff.uni-marburg.de
Inhalt ausklappen Inhalt einklappen Publications of Project 1
Walter E, Vielmuth F, Wanuske MT, Seifert M, Pollmann R, Eming R, Waschke J. Role of Dsg1- and Dsg3-Mediated Signaling in Pemphigus Autoantibody-Induced Loss of Keratinocyte Cohesion. J Front Immunol. 2019
Kugelmann D, Rötzer V, Walter E, Egu DT, Fuchs MT, Vielmuth F, Vargas-Robles H, Schnoor M, Hertl M, Eming R, Rottner K, Schmidt A, Spindler V, Waschke Role of Src and Cortactin in Pemphigus Skin Blistering. J. Front Immunol. 2019;10:626
Amber KT, Maglie R, Solimani F, Eming R, Hertl M. Targeted Therapies for Autoimmune Bullous Diseases: Current Status. Drugs. 2018;78(15):1527-1548Pollmann R, Eming R. Research Techniques Made Simple: Mouse Models of Autoimmune Blistering Diseases. J Invest Dermatol. 2017;137(1):e1-e6
Walter E, Vielmuth F, Rotkopf L, Sárdy M, Horváth ON, Goebeler M, Schmidt E, Eming R, Hertl M, Spindler V, Waschke. Different signaling patterns contribute to loss of keratinocyte cohesion dependent on autoantibody profile in pemphigus. J. Sci Rep. 2017;7(1): 3579





